|
 |
- Search
| Asian J Kinesiol > Volume 24(3); 2022 > Article |
|
Abstract
OBJECTIVES
To investigate the relationships among intramuscular cooling rates during (IM cooling rate) and after cold water immersion (CWI) (Post-IM cooling rate), skin tissue cooling rate during CWI (skin cooling rate), and anthropometric characteristics, and develop prediction models to assist clinical decision making.
METHODS
After a 30-min cycling trial, 16 young healthy adults received a CWI treatment (10 ┬░C) until either intramuscular thigh temperature (2 cm sub-adipose) of the rectus femoris decreased 7 ┬░C below preexercise level or 30 minutes was reached. Temperatures were recorded using skin and implantable fine-wire thermocouples. Before the cycling trial, %BF, anterior thigh adipose tissue thickness, muscle thickness, total thigh volume, and thigh circumference were measured. PearsonŌĆÖs correlation coefficients were used to determine significant predictors of IM and Post-IM cooling rates (cooling rate: the amount of temperature reduction per minute). All predictors, including skin cooling rate, %BF, adipose tissue thickness, muscle thickness, total thigh volume, and thigh circumference, were included in multiple linear regression models to figure out factors that best predict the IM and Post-IM cooling rates.
RESULTS
Correlation analysis demonstrated significant correlations between IM cooling rate and skin cooling rate (r=.85), %BF (r=-.79), and adipose tissue thickness (r=-.79), and between Post-IM cooling rate and thigh circumference (r=-.68), adipose tissue thickness (r=-.58), total thigh volume (r=-.56), and %BF (r=-.53). Regression models identified skin cooling rate and %BF to have the greatest predictability for IM cooling rate (R2 =.82) and muscle thickness and thigh circumference to have the greatest predictability for the Post-IM cooling rate (R2 =.68).
CONCLUSIONS
This study provides justification for the use of skin cooling rates during CWI and %BF to estimate IM cooling rate and muscle thickness and thigh circumference to estimate Post-IM cooling rate. These findings will help practitioners to determine the duration of CWI treatment after exercise.
Cryotherapy, the therapeutic use of cold, has been widely used for treating exercise induced muscle damage (EIMD), typified by disruption of the sarcolemma, fragmentation of the sarcoplasmic reticulum, lesions of the plasma membrane, cytoskeletal damage, and swollen mitochondria [1,2]. Especially, cold water immersion (CWI) has been used for athletic recovery or injury rehabilitation to reduce tissue temperature, which in turn decreases swelling by slowing blood flow to the affected area, pain by reducing nerve conduction velocity, and secondary hypoxia and cell damage by suppressing cellular metabolism [3,4]. Given the potential detrimental impact of intense training and competition on athletic well-being, effective post-exercise recovery procedures are vital for optimal performance, and thus CWI has been utilized to enhance recovery and hasten return to optimal performance capabilities.
CWI has gained an overwhelming amount of anecdotal support as well as empirical evidence for the treatment of various indices of EIMD [5]. Several studies used CWI protocols of 8-10┬░C [6ŌĆō8], and only one of these studies reported improvements in markers of EIMD, including decreased myoglobin, soreness, and increased muscle contraction force [6]. Research has demonstrated that cellular metabolism is halved when tissue is cooled by 7-10┬░C [9]; however, previous CWI protocols (10 ┬▒ 2┬░C, 10 ┬▒ 2 min) reduced the intramuscular temperature by 1.61┬░C at a depth of 3 cm, 3.65┬░C at 2 cm, and 6.40┬░C at 1 cm [10], which indicates the protocols were likely insufficient to cause adequate tissue cooling to induce the proper amount of tissue metabolism.
The transfer of heat from one body part to another depends on several factors, such as metabolic activity and perfusion, treatment duration, temperature gradient, the relative mass of the bodies, size of contact area, depth of the desired cooling, and the heat capacity of each material [11,12]. Specifically, body composition influences temperature changes during and after CWI [13]. During CWI, adipose tissue provides insulating effects, which slows the temperature reduction, and skeletal muscles generate heat (i.e., thermogenesis) to compensate for heat loss [12,14]. Thus, the amount of adipose tissue and intramuscular temperature changes during and after CWI present a significant inverse relationship [12]. Also, although heat transfer is reversed after a CWI treatment ceases, intramuscular temperatures continue to decrease awhile (i.e., afterdrop) [12]. However, no studies have additionally examined the role of muscle thermogenesis on intramuscular temperature changes in these phases. Thus, amounts of both adipose tissue and muscle should be taken into account in CWI protocols.
Morphological characteristics also have a significant impact on tissue cooling rate [15]. Specifically, the body surface area to mass ratio has a significant effect on the cooling rate, and a larger body better defends tissue temperature [15]. Considering the importance of morphological characteristics as well as the roles of both adipose tissue and muscle on tissue cooling, the total volume of the body part immersed in cold water should be additionally considered in CWI protocols.
Rupp et al. reported that CWI at 12┬░C decreases 1-cm intramuscular temperature of the gastrocnemius muscle by 8┬░C after 39.91 minutes [16]. However, the time to the target cooling temperature is much longer than in other previous trials [10], and the result from the gastrocnemius may not be applicable to determine treatment parameters on other muscles with a greater volume (e.g., rectus femoris) due to multiple factors of heat transfer and thermogenesis. Further, a prior study regarding the predictive ability of anthropometric characteristics, such as including percentage body fat (%BF), thigh length, muscle thickness, total thigh volume, thigh circumference, and adipose tissue thickness, on intramuscular cooling during CWI has not considered the predictive ability of skin cooling rate during CWI, which can be easily utilized in clinical settings [17]. Therefore, the aims of this study were to (1) to examine the relationships among intramuscular cooling rates of the rectus femoris during (IM cooling rate) and after post-exercise CWI (Post-IM cooling rate), skin tissue cooling rate during CWI (skin cooling rate), and anthropometric characteristics, including percentage body fat (%BF), anterior thigh adipose tissue thickness, muscle thickness, total thigh volume, and thigh circumference, and (2) to develop multiple regression models using skin cooling rate and anthropometric factors for predicting IM and Post-IM cooling rates and assisting clinical decision making. In this study, the cooling rate was defined as the amount of temperature reduction per minute. Further, these anthropometric characteristics were selected based on a previous study [17], and a skin tissue cooling rate was additionally included in the analysis. We hypothesized that adipose tissue thickness, muscle thickness, %BF, total thigh volume, and thigh circumference will present inverse relationships, and skin cooling rates will display a direct relationship with IM and Post-IM cooling rates.
Sixteen healthy young adults (nationality: America, race: white, society: collegiate students) were recruited for this descriptive laboratory study. Eligibility criteria included no lower extremity injury, no neurophysiological conditions affecting normal tissue thermodynamics, and ability to pedal on a stationary ergometer for 30 minutes at moderate-to-hard intensity. They self-reported physical activity levels ranging from 1 to 2 days of light-intensity exercise per week to 5 to 6 days of moderate- to high-intensity exercise per week. An a-priori power analysis (╬▒ = 0.05, 1-╬▓ = 0.80) utilizing data from a previous study was conducted with G*Power (Version 3.1.9.2 Kiel University, Germany) [18], and we estimated a sample size of 13 subjects would be necessary to allow us sufficient power (1-╬▓ = 0.8). Each subject provided written informed consent before participating in the study. The study protocol was approved by the university institutional review board (IRB-4065).
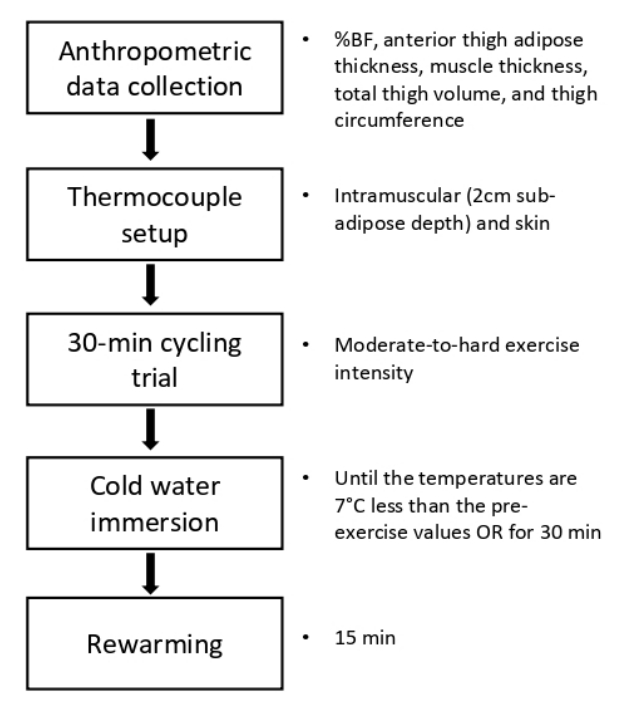
Subjects were familiarized with all procedures prior to testing. Each subject reported to the University Sports Medicine Research Center and refrained from consuming alcohol, caffeine, or food for 1 hour and from any vigorous activities for 2 hours before the test to stabilize extremity blood flow. The experimental procedure was presented in <Figure 1>. Prior to the CWI, the following anthropometric variables were examined: %BF, anterior thigh adipose tissue thickness, muscle thickness, total thigh volume, and thigh circumference.
%BF was calculated using the Jackson and Pollack three site measurement [19], which accurately estimates body composition when compared to hydrostatic weighing (r = 0.92) and can be easily utilized in clinical settings. Anterior thigh adipose tissue and muscle thickness were estimated based on the depths of adipose-muscle and muscle-bone interfaces using BodyMetrix A-Mode ultrasound (InteleMetrix, Livermore, CA, USA). Using thigh length (h), thigh circumference (CT), and anterior thigh adipose thickness (T) as input variables, total thigh volume was estimated using a series of equations suggested by Tothill and Steward based on geometric principles assuming a circular cross-section as follows [17,20]:
This method has been proven to be highly accurate compared to magnetic resonance imaging. Thigh circumference was measured at the midpoint between 1 cm superior to the patella and the greater trochanter [20].
Before measuring intramuscular and skin temperature, the midpoint was marked on the anterior thigh to indicate the location of insertion of the temperature probe. Thereafter, a 10cm2 area surrounding the midpoint mark was cleaned with povidone-iodine swabs. The insertion site, 1 cm superior to the midpoint mark, was anesthetized using 1 ml of Bupivacaine (Hospira, Inc., Lake Forest, IL, USA). A tube surrounding the probe insertion catheter (18 gauge, 8.89 cm; PSS World Medical, Jacksonville, FL, USA) was cut to permit the catheter to reach a depth of 2 cm sub-adipose tissue. Once the catheter reached the standard depth, an IT-18 implantable thermocouple (Physitemp Instruments Inc, Clifton, NJ, USA) was fed through the catheter. The pressure was maintained on the thermocouple wire as the catheter was removed from the thigh to ensure that it remained at the correct depth. After removing the catheter, the thermocouple wire was taped to the thigh using a Cover Roll dressing (BSN Medical, Hamburg, Germany).
The surface thermocouple, which recorded skin temperature, was attached to the anterior thigh 1 cm inferior to the midpoint mark and waterproofed using a 8 x 8 cm strip of transparent waterproof adhesive film (OpSite; Smith and Nephew, Largo, FL, USA). The edges of the OpSite were secured with adhesive stretch tape (Cover-Roll Stretch; BSN Medical, Hamburg, Germany). The leads were attached to the hip (1 cm superior to the iliac crest) using the adhesive stretch tape to prevent the leads from being disturbed. Then, the thermocouples were connected to an 8-channel Thermes thermocouple data-acquisition device (accuracy = ┬▒0.1┬░C, 0.2%; Physitemp Instruments, Clifton, NJ, USA), and intramuscular and skin temperature was recorded with a sample rate of 2 Hz from the onset of the 30-min cycling trial to the end of the 15-min rewarming period after CWI. Intramuscular skin temperature measurements using our methods and equipment have been tested previously with high reported intrarater reliability (ICC = .91) and interrater reliability (ICC = .97). Similarly, previous validity tests using the Physitemp thermocouples used in the current study were high based a PearsonŌĆÖs product correlation with a reported r = .97 [21].
Subjects then performed a 30-min cycling trial on a stationary cycle ergometer (Freemotion Fitness, Colorado Springs, CO, USA), and heart rate was continuously monitored using a pulse oximeter (Nellcor Puritan Bennett Inc., Pleasanton, CA, USA). Heart rate was maintained between 130 and 150 beats per minute during the trial, which signifies an age-specific moderate-to-hard exercise intensity [22]. Immediately after the trial, subjects were immersed in cold water (10┬░C) up to the level of the iliac crest in a temperature-regulated cold plunge pool (Classic; HydroWorx). Water temperature at 10┬░C was selected as it is in the mid-range of CWI temperatures used in the literature [23]. Temperature recordings continued until both intramuscular and surface temperatures are 7┬░C less than their respective pre-exercise values as tissue metabolism declines by 50% with a decrease in temperature of 7-10┬░C [9]. If any temperature was not decreased by 7┬░C, the subject was removed from the CWI protocol after 30 minutes to prevent potential hypothermia [24].
Following the CWI treatment, the subjects dried off and stood in a temperature-controlled environment (21.78C). When the intramuscular temperature began to increase, the recording was continued for 15 minutes (15-min rewarming period) and then stopped. After the rewarming period, the thermocouples were withdrawn, and insertion depth was remeasured to check if the thermocouple remained at the correct depth. The insertion site was cleansed with an alcohol wipe and bandaged. Sterile techniques were used during all invasive procedures. Cooling rates (intramuscular and skin) during and after CWI were calculated using the following equations.
Pearson product-moment correlation was performed to examine the relationship among IM cooling rate, Post-IM cooling rate, skin cooling rate, and anthropometric characteristics, including %BF, adipose tissue thickness, muscle thickness, total thigh volume, and thigh circumference.
Then, a backward elimination, multiple linear regression was conducted using anthropometric characteristics and skin cooling rate to determine variables that best predict IM and Post-IM cooling rates. To test the multicollinearity assumption, the variance inflation factor and tolerance were examined. The alpha level was set to 0.05, and all statistical analyses were performed in RStudio (version 1.2.5033).
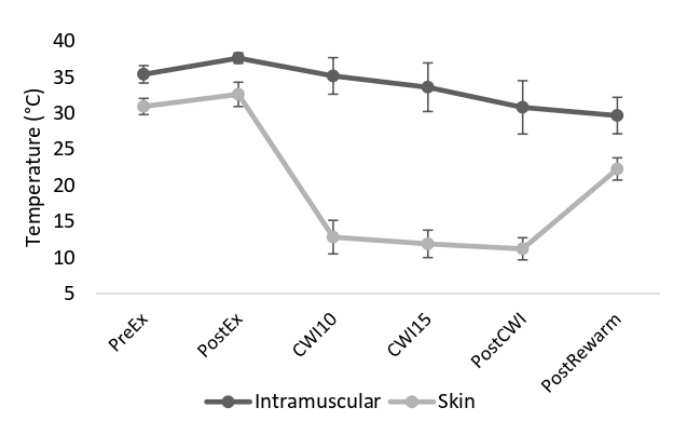
The demographic and anthropometric characteristics across all subjects are presented in <Table 1>. Intramuscular and skin temperatures at different time points, including pre-exercise, post-exercise, 10 and 15 min in CWI, post-CWI, and end temperature, were presented in <Figure 2>. Only three subjects presented a 7-degree decrease in both IM and skin temperature during CWI. The intramuscular temperatures in all subjects continued to decrease after the termination of the CWI treatment, whereas skin temperature immediately increased.
Significant correlations were observed between IM cooling rate and skin cooling rate, IM cooling rate and %BF, IM cooling rate and adipose tissue thickness, Post-IM cooling rate and thigh circumference, Post-IM cooling rate and adipose tissue thickness, Post-IM cooling rate and total thigh volume, and Post-IM cooling rate and %BF. Correlation coefficients between intramuscular cooling rates and all predictor variables are presented in <Table 2>.
Backward regression models ascertained several significant predictors for the IM and Post-IM cooling rates. For the IM cooling rate, skin cooling rate and %BF explained approximately 82% of the variability (r = .90, R2 = .82, F2,13 = 28.71, P < .01). For the Post-IM cooling rate, muscle thickness and thigh circumference explained approximately 68% of the variability (r = .83, R2 = .68, F2,13 = 14.10, P < .01). The models had variance inflation factors of 1.0 and tolerance of 1.0. A summary of the regression analyses for IM and PostIM cooling rates is presented in <Table 3>, and predictive equations for IM cooling rate and Post-IM cooling rate are as follows:
IM cooling rate had a significant positive correlation with skin cooling rate and significant negative correlations with %BF and adipose tissue thickness. Post-IM cooling rate had significant negative correlations with thigh circumference, adipose tissue thickness, total thigh volume, and %BF. To predict IM cooling rate, skin cooling rate and %BF played the largest role, while the muscle thickness and thigh circumference played the largest role to predict Post-IM cooling rate.
To effectively treat acute muscular damage, a proper amount of cooling of the tissue is necessary. As thigh musculature is cooled, deeper tissues are cooled by losing heat to more superficial tissues. This can be thought of as heat being passed from deep tissues out to the cold modality which is the final heat acceptor. Adipose tissue acts as an inhibitor to the transfer of heat. This means that individuals with a greater amount of adipose tissue will take longer to cool intramuscularly, which agrees with previous research indicating a significant inverse relationship between adipose tissue thickness and intramuscular cooling [12,25]. Further, in the current study, the subset that reached adequate cooling (7┬░C below the pre-exercise temperature) had a lower average adipose tissue thickness (0.8 cm) than the group that did not (1.2 cm). Specifically, Otte et al. accentuated that as thigh adipose tissue thickness increases, the ability for intramuscular tissue to cool to the recommended 7┬░C below baseline within a normal treatment time diminishes [25].
A variable not considered by previous trials was %BF. The results of the current study show that %BF and anterior thigh adipose thickness were similarly correlated with IM cooling rate. As a single predictor, %BF explained 63% of the variance of IM cooling rate compared to 62% explained by adipose tissue thickness, which reinforces that the amount of adipose tissue overlying a target muscle has a significant negative effect on intramuscular cooling. Considering the predictive ability of the skin cooling rate and the significant correlation with IM cooling rate, CWI protocols based on the skin cooling rate as well as %BF will most accurately cool target tissues to the recommended temperature. This finding agrees with a previous prediction model which also includes both skin temperature and adipose tissue thickness (skinfold) to predict intramuscular temperature during CWI [18].
To the best of our knowledge, this was the first experimental study investigating the correlation between muscle thickness and tissue cooling rates during and after CWI. When exposed to cold, shivering thermogenesis from skeletal muscles is the primary defense mechanism to compensate for heat loss when muscle contractions through voluntary movements are not available [14]. The regression model suggested muscle thickness as one of the most impactful predictor variables for Post-IM cooling rate, and we presumed that the thicker muscle may produce more thermogenesis, which, in turn, has a greater impact on the IM cooling rate. Heat is a by-product of exothermic biochemical reactions, in which combined carbohydrate, lipids, and protein oxidation act as necessary substrates to sustain shivering thermogenesis [14]. As shivering thermogenesis intensifies, the contribution of carbohydrates progressively increases whereas those of lipids and proteins decrease. Further, 75-80% of the total glucose for sustaining shivering thermogenesis is supported by muscle glycogen reserves [14]; thus, it was presumed that the thicker muscle may contain more glycogen and produce more heat. Therefore, future studies may wish to consider the metabolic requirements of shivering thermogenesis and the impacts on tissue cooling during cryotherapy.
The intramuscular temperature continued to decrease after CWI. Interestingly, four subjects had a greater intramuscular temperature decrease after the CWI treatment than during CWI. According to the correlation analysis, thigh circumference and total thigh volume demonstrated significant inverse relationships with Post-IM cooling rate. According to literature, surface area to mass ratios can have a significant effect on cooling rate [15], which in that implies the intramuscular temperature of larger thighs is less impacted by external temperature (e.g., cold water and warm ambient environment). Further, although both adipose tissue thickness and %BF showed significant negative correlations with Post-IM cooling rate, none of the variables were included in the prediction model for Post-IM cooling rate. Thus, future trials may wish to figure out the significant factors of Post-IM cooling rate.
According to literature, crushed ice has normally been reported to produce a greater magnitude of cooling in a given time than CWI as it has a greater temperature gradient than CWI and more potential to extract heat from the tissue [26]. Whereas the majority of the subjects in the current study did not reach the adequate cooling of 7┬░C below pre-exercise temperature within 30 min, a crushed-ice bag treatment cooled the same muscle (i.e., rectus femoris) to 7 ┬░C below baseline in approximately 25 min [27]. A study by Myrer et al., presenting comparable results, reported that the intramuscular temperature began to increase immediately following the termination of the ice pack treatment while the CWI treatment continued to cool the muscle for approximately 25 min with a total post-treatment reduction of 1.8 ┬▒ 1.4┬░C [28]. Rupp et al also reported intramuscular temperature remained significantly lower at 90 min post-CWI than the crushed ice bag treatment [16]. The current study also demonstrated a significant post-CWI reduction of intramuscular temperature of 1.7 ┬▒ 1.0┬░C over 23 ┬▒ 22.5 min. The results identify that while ice packs produce a lower intramuscular temperature during treatment, the sustained temperature reductions brought about by CWI may be more effective at providing adequate cooling. Thus, future trials and treatments should consider the post-treatment effects of CWI.
Our results indicate that the average intramuscular temperature reductions after 10 and 15 min in CWI were 2.5 ┬▒ 2.3┬░C and 4.0 ┬▒ 3.0┬░C respectively. Minimal decreases in intramuscular temperature are achieved after 10-15 min of CWI, which is the length of CWI normally used in clinical settings. Thus, it is evident that studies to date have not been using protocols that elicit a sufficient temperature reduction that is needed to reduce tissue metabolism by 2 to 3 fold. Further, CWI after an acute injury, using previous clinical protocols, will likely not yield sufficient temperature reductions necessary to prevent secondary hypoxic injury. Therefore, parameters for CWI need to be set based on the purpose of each treatment, and practitioners in various clinical settings may wish to utilize the predictive equations suggested in the current study for estimating IM and Post-IM cooling rates in future CWI treatments.
Limitations of the current study include an absence of further experimental groups to compare the effects of CWI at different temperatures and an absence of various indices of EIMD. Therefore, future research to figure out the optimal post-workout CWI parameters to reduce the symptoms of EIMD is needed. Further, future studies comparing a general CWI duration of 10-15 min with a duration based on time to cool 7┬░C below baseline temperature on indices of EIMD may enhance our understanding of how to cool muscle for post-workout recovery purposes.
IM cooling rate was highly correlated with the skin cooling rate during CWI, %BF, and adipose tissue thickness, and Post-IM cooling rate was highly correlated with the adipose tissue thickness, total thigh volume, and %BF. Further, to predict IM cooling rate, skin cooling rate and %BF can be used as predictors, and muscle thickness and thigh circumference can be used as predictors of Post-IM cooling rate. Practitioners may need to use the prediction models to determine the parameters of CWI treatments to induce the proper amount of intramuscular cooling during and after the CWI treatments.
Figure┬Ā2.
Intramuscular and skin temperature (┬░C) (mean, SD). PreEx: temperature before the cycling trial; PostEx: temperature after the cycling trial; CWI10; after 10 min in cold water immersion; CW15: after 15 min in cold water immersion; PostCWI: immediately after cold water immersion; PostRewarm: after the 15-min rewarming period.
Table┬Ā1.
Demographic and anthropometric characteristics
Table┬Ā2.
Correlation coefficients (r[p]) between intramuscular cooling rates, anthropometric characteristics, and skin cooling rate during CWI
Table┬Ā3.
Summary of regression analyses for intramuscular cooling rates during and after CWI
References
1. Nogueira NM, Felappi CJ, Lima CS, Medeiros DM. Effects of local cryotherapy for recovery of delayed onset muscle soreness and strength following exercise-induced muscle damage: systematic review and meta-analysis. Sport Sci Health. 2020; 16(1): 1ŌĆō11.

2. Eston R, Peters D. Effects of cold water immersion on the symptoms of exercise-induced muscle damage. Journal of Sports Sciences. 1999; 17(3): 231ŌĆō8.


3. Algafly AA, George KP. The effect of cryotherapy on nerve conduction velocity, pain threshold and pain tolerance. British Journal of Sports Medicine. 2007; 41(6): 365ŌĆō9.



4. Swenson C, Sw├żrd L, Karlsson J. Cryotherapy in sports medicine. Scandinavian Journal of Medicine & Science in Sports. 1996; 6(4): 193ŌĆō200.


5. Burgess TL, Lambert MI. The efficacy of cryotherapy on recovery following exercise-induced muscle damage : invited review article. International SportMed Journal. 2010; 11(2): 258ŌĆō77.
6. Bailey DM, Erith SJ, Griffin PJ, Dowson A, Brewer DS, Gant N, Williams C. Influence of cold-water immersion on indices of muscle damage following prolonged intermittent shuttle running. Journal of Sports Sciences. 2007; 25(11): 1163ŌĆō70.


7. Roberts LA, Raastad T, Markworth JF, Figueiredo VC, Egner IM, Shield A, Cameron-Smith D, Coombes JS, Peake JM. Post-exercise cold water immersion attenuates acute anabolic signalling and long-term adaptations in muscle to strength training. The Journal of Physiology. 2015; 593(18): 4285ŌĆō301.



8. Mawhinney C, Low DA, Jones H, Green DJ, Costello JT, Gregson W. Cold Water Mediates Greater Reductions in Limb Blood Flow than Whole Body Cryotherapy. Med Sci Sports Exerc. 2017; 49(6): 1252ŌĆō60.


9. Meeusen R, Lievens P. The Use of Cryotherapy in Sports Injuries. Sports Medicine. 1986; 3(6): 398ŌĆō414.


10. Freitag L, Clijsen R, Deflorin C, Taube W, Taeymans J, Hohenauer E. Intramuscular Temperature Changes in the Quadriceps Femoris Muscle After Post-Exercise Cold-Water Immersion (10┬░C for 10 min): A Systematic Review With Meta-Analysis. Frontiers in Sports and Active Living. 2021; 3:100.



11. Merrick MA, Jutte LS, Smith ME. Cold Modalities With Different Thermodynamic Properties Produce Different Surface and Intramuscular Temperatures. J Athl Train. 2003; 38(1): 28ŌĆō33.


12. Myrer WJ, Myrer KA, Measom GJ, Fellingham GW, Evers SL. Muscle Temperature Is Affected by Overlying Adipose When Cryotherapy Is Administered. J Athl Train. 2001; 36(1): 32ŌĆō6.


13. Stephens JM, Halson SL, Miller J, Slater GJ, Chapman DW, Askew CD. Effect of Body Composition on Physiological Responses to Cold-Water Immersion and the Recovery of Exercise Performance. International Journal of Sports Physiology and Performance. 2018; 13(3): 382ŌĆō9.


14. Haman F, Blondin DP. Shivering thermogenesis in humans: Origin, contribution and metabolic requirement. Temperature. 2017; 4(3): 217ŌĆō26.



15. Stocks JM, Taylor NAS, Tipton MJ, Greenleaf JE. Human Physiological Responses to Cold Exposure. Aviation, Space, and Environmental Medicine. 2004; 75(5): 444ŌĆō57.

16. Rupp KA, Herman DC, Hertel J, Saliba SA. Intramuscular Temperature Changes During and After 2 Different Cryotherapy Interventions in Healthy Individuals. Journal of Orthopaedic & Sports Physical Therapy [Internet]. 2012 Aug 1 [cited 2022 Feb 25]; Available from: https://www.jospt.org/doi/abs/10.2519/jospt.2012.4200.
17. Rech N, Bressel E, Louder T. Predictive Ability of Body Fat Percentage and Thigh Anthropometrics on Tissue Cooling During Cold-Water Immersion. Journal of Athletic Training. 2020; 56(6): 548ŌĆō54.



18. Jutte LS, Merrick MA, Ingersoll CD, Edwards JE. The relationship between intramuscular temperature, skin temperature, and adipose thickness during cryotherapy and rewarming. Archives of Physical Medicine and Rehabilitation. 2001; 82(6): 845ŌĆō50.


19. Jackson AS, Pollock ML. Practical Assessment of Body Composition. The Physician and Sportsmedicine. 1985; 13(5): 76ŌĆō90.

20. Tothill P, Stewart AD. Estimation of thigh muscle and adipose tissue volume using magnetic resonance imaging and anthropometry. Journal of Sports Sciences. 2002; 20(7): 563ŌĆō76.


21. Selkow NM, Day C, Liu Z, Hart JM, Hertel J, Saliba SA. Microvascular Perfusion and Intramuscular Temperature of the Calf During Cooling. Med Sci Sports Exerc. 2012; 44(5): 850ŌĆō6.



22. Swain DP, Abernathy KS, Smith CS, Lee SJ, Bunn SA. Target heart rates for the development of cardiorespiratory fitness. Med Sci Sports Exerc. 1994; 26(1): 112ŌĆō6.


23. Vromans BA, Thorpe RT, Viroux PJ, Tiemessen IJ. Cold water immersion settings for reducing muscle tissue temperature: a linear dose-response relationship. J Sports Med Phys Fitness. 2019; 59(11): 1861ŌĆō9.


24. Tipton MJ, Collier N, Massey H, Corbett J, Harper M. Cold water immersion: kill or cure? Experimental Physiology. 2017; 102(11): 1335ŌĆō55.


25. Otte JW, Merrick MA, Ingersoll CD, Cordova ML. Subcutaneous adipose tissue thickness alters cooling time during cryotherapy. Archives of Physical Medicine and Rehabilitation. 2002; 83(11): 1501ŌĆō5.


26. Bleakley CM, Hopkins JT. Is it possible to achieve optimal levels of tissue cooling in cryotherapy? Physical Therapy Reviews. 2010; 15(4): 344ŌĆō50.