Introduction
Sarcopenia is a process of aging that means a decrease in muscle strength and function due to a decrease in skeletal muscle [1]. From a pathophysiologic perspective, sarcopenia can be considered an organ failure which can develop chronically or acutely [2]. Clinical studies indicate that sarcopenia is associated with a decreased walking ability, more frequent use of a cane, and difficulty in climbing, lifting, kneeling, or standing up from a chair [3-4]. Sarcopenia is a syndrome a risk of adverse outcomes such as physical disability, poor quality of life and death. In this research, we reviewed the literature to introduce the definitions and diagnostic criteria, pathophysiology and management of sarcopenia with clinical relevance for exercise physiologist. This study is designed to introduce the latest diagnostic methods of sarcopenia and is intended to help the elderly to organize exercise programs. The development of criteria for the diagnosis of sarcopenia is complex and must depend upon skeletal muscle mass and functional capacity in geriatric patients, this should draw health care provider. In addition, the effect of exercise being introduced as a preventive measure of sarcopenia is presented.
Definitions
Sarcopenia was first coined by Irwin Rosenberg in 1989 is now widely accepted to describe the steady and involuntary loss of skeletal muscle mass during aging [8]. Although many challenges exist to make definition of sarcopenia, but until now, there is no consensus. Baumgartner et al. (1998) were the first define sarcopenia as the appendicular skeletal muscle/height2 (ASM/h2) by measuring muscle mass as DXA (dual-energy X-ray absorptiometry) and set the case of less than two standard deviation of young healthy mean as the set-off point of sarcopenia [1]. Second definition of sarcopenia was developed by Janssen and colleagues. They measured SMI (%; total SMI [kg]/weight [kg]├Ś100) using BIA (bio-electrical impedance analysis) [5]. Newman et al. (2003) indicated that the ASM/height2 index primarily identified individuals with low BMI as sarcopenia [6]. Cruz-Jentoft et al. (2010) measured muscle mass as DXA and BIA, cut-off point was same as Baumgartner et al. (1998). Additionally, muscle strength and physical performance were evaluated as grip strength and gait speed or SPPB (short physical performance battery) [3]. Fielding et al. (2011) measured muscle mass using only DXA, and separated men (Ōēż7.23kg/m2) and womenŌĆÖs standards (Ōēż5.67kg/m2) with appendicular lean mass/height2 (ALM/h2). It was defined as physical function was evaluated as gait speed but did not provide a standard for muscle strength [7].
In 2010, the European Working Group on Sarcopenia in Older People (EWGSOP) and the Foundation for the National Institutes of Health (FNIH) established sarcopenia definitions and diagnostic criteria [8]. EWGSOP defined sarcopenia as a gradual decrease in skeletal muscle mass and strength, and reduced muscle mass, strength and physical performance became diagnostic criteria [8]. The International Working Groups on Sarcopenia (IWGS) [7] and the EWGSOP [8] adopted the criterion based on DXA to define sarcopenia by Baumgartner et al (1998) [1].
In 2019, a revised EWSGOP2 has developed new consensus criteria [9]. In EWGSOP2, muscle mass is measured as BIA to calculate ASM/h2 and body mass index and muscle strength is evaluated by hand grip strength. What makes EWGSOP2 different from EWGSOP is that it categorizes the severity of sarcopenia and sets new cut-off points [10].
Meanwhile, as Asia differs from Europe by ethnicities, cultural and lifestyles, diagnostic criteria of Asian Working Group for Sarcopenia (AWGS) different from EWGSOP are introduced [11]. In AWGS, muscle mass was measured as DXA and BIA and the result calculated as skeletal muscle mass/h2 was used as an evaluation variable, and muscle strength was evaluated as hand grip strength in the same way as EWGSOP. The AWGS was also revised in 2019, and criteria for questionnaire and calf circumference were introduced to help people easily recognize the risk of sarcopenia, and in addition, diagnostic methods such as 6-minute walking, SPPB, and chair stand test were defined [12].
Numerous epidemiological studies using different methods of measurement and cut-off points have attempted to establish the prevalence of sarcopenia. In general, 5-13% of population aged 60-70 years and 11-50% of population in their 80s have been assumed to have sarcopenia. Thus, 3.6 million persons in the USA were estimated to be with sarcopenia [13-16].
Diagnostic Criteria
Various methods were introduced to assess sarcopenia. Measuring skeletal muscle mass is a very direct and useful way to assess sarcopenia. BIA and anthropometry are also very useful method and popular one to assess sarcopenia, because of the noninvasiveness and absence of radiation hazard. These electrical equipment measures electrical resistance, skinfold thickness and circumferences. These factors make it possible to predict muscle mass. However, the limitation is its vulnerability for hydration and systemic error while collecting data.
Recently, DXA, magnetic resonance imaging (MRI), and computed tomography (CT) were suggested to assess whole body skeletal muscle. However, these contain limitation of radiation exposure and cost issue [16].
Baumgartner et al. (1998) developed the operational definition of sarcopenia first [1]. The approach was defined as ASM (sum of the masses of arm and leg lean soft tissue from DXA) divided by height squared (also referred to as stature, ASM/S2). They used statistics to get cutoff values. The two standard deviation below sex-specific mean of the distributions in a reference sample of young and middle-aged adults from the Rosetta Study was the criteria [14]. Other studies reported sarcopenia prevalence in different age, racial and gender characteristics, using various method [17-18]. For example, Melton et al. (2000) proposed different sarcopenia index defined as total lean body mass/stature2 [18], Janssen et al. (2004) used BIA to calculate the index defining total skeletal muscle/stature2 in US population [5].
In EWGSOP2, ASM/h2 and body mass index (ASM/BMI) calculated as BIA, and cut-off was set to <7.0kg/m2 for men and <5.5kg/m2 for women. In addition, cut-off point was set to ASM/BMI ratio less than <0.789 for men and <0.512 for women. On the other hand, muscle strength was evaluated as grip strength, and cut-off point was set to <27kg for men and <16kg for women [11]. Gait speed is used to classify the severity of sarcopenia [10].
AWGS evaluates muscle mass as DXA and BIA. Cut-off point of DXA is set to less than <7.0kg/m2 for men and <5.4kg/m2 for women. BIA was calculated as skeletal muscle mass/h2 and set to less than <7.0kg/m2 for men and <5.7kg/m2 for women. The cut-off point of grip strength is <26kg for men and <18kg for women [12]. The criteria for calf circumference were <34 cm for men and <33cm for women, and the score criteria for questionnaires were also presented (SARC-F Ōēź 4 or SARC-CalF Ōēź 11). Finally, the modified AWGS (2019) should not only diagnose sarcopenia, but also monitor its potential [12].
Over the years, no relevant consensus has been reached to find the definition and diagnostic criteria of sarcopenia until now. However, the fact that the best measures are based on muscle strength rather than muscle mass particularly in the context of cardiovascular disease has been confirmed [19].
Pathophysiology of Skeletal Muscle Loss
Sarcopenia can cause various clinical problems, such as loss of strength, mobility disorders, disability, and poor quality of life [4, 10, 20-22]. Various factors including vitamin D deficiency, pro-inflammatory cytokines, decreasing growth hormones etc., can cause sarcopenia [7].
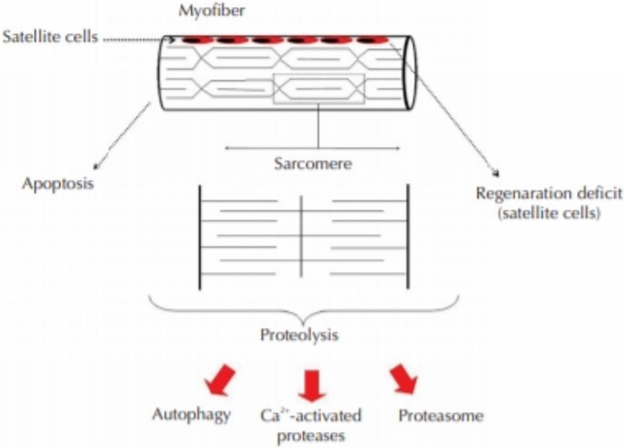
The role of apoptosis in post-mitotic tissues such as skeletal muscle is not clearly understood [23]. From the cellular level, studies proved that different conditions leading to muscle wasting involved different cell signaling pathways that might lead to programmed cell death (apoptosis), increased protein breakdown, or even decreased activation of the satellite cells responsible for muscle regeneration (Figure 1).
Mauro (1961) observed a group of mononucleated cells at the periphery of adult skeletal muscle myofibers by electron microscopy. These were named satellite cells due to their sublaminar location and intimate association with the plasma membrane of myofibers. The direct juxtaposition of satellite cells and myofibers immediately raised a hypothesis that these cells may be involved in skeletal muscle growth and regeneration [24]. Homeostatic and regenerative replacement of skeletal muscle fibers requires the activity of satellite cells. Satellite cell function is controlled by both intrinsic and extrinsic regulatory cues, and pathological deregulation of satellite cell function has been proposed to play an important role in age-dependent deterioration of muscle function and in muscle dystrophic disease. However, satellite cell transplantation to enhance muscle repair or restoration has little data in the literature.
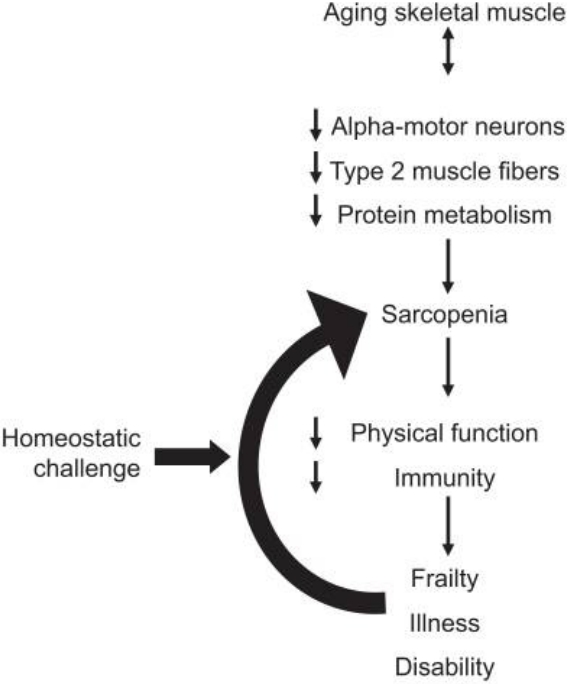
It is very clear that sarcopenia is positively associated with various physical and physiological problem, but the problem is that it is very hard to know which one comes first. However, aging is the prime factor for this condition. Recent studies described the detailed mechanism of the sarcopenia in the elderly and found that one of the most prominent causes of sarcopenia is inactivity [25] (Figure 2). Exercise stimulates the release of muscle growth factors to activate satellite cells and protein synthesis. The regenerated muscle can delay the aging process. In other word, aging process accompanied by sarcopenia can be accelerated by immobilization. Sarcopenia can be aggravated by itself, because of the decreased physical function can also cause sarcopenia.
Management
In recent years, there has been an increased interest in the treatment and management of sarcopenia. The management of sarcopenia should focus on improving muscle mass and physical function. Researchers have confirmed that nutritional support, medication, and physical exercise play important roles in the treatment of sarcopenia. Consuming the proper amounts of dietary protein and physical exercise may lead to a reduction in muscle loss and physical function.
Protein and Amino Acid Supplementation
Nutrition is the first key element as for treatment and prophylaxis to solve sarcopenia. Morley suggested daily intake of 1.2~1.5 g/kg of protein to prevent sarcopenia [16]. Recent randomized controlled trial indicates that it is important to ingest a sufficient amount of high-quality protein (25-30g) with each meal rather than one large bolus [26]. Several studies revealed that the nonessential amino acids are also required in a nutritional supplement to stimulate muscle protein anabolism [27], because the essential amino acid leucine increases protein anabolism and decreases protein breakdown [28]. Researches are needed to be determine if the quality of the dietary protein and amino acid supplementation enhance skeletal muscle. In addition, the mechanism of action of the effect of protein and amino acid support with physical exercise on skeletal muscle and muscle strength.
Medication
Medication is another important regime to manage sarcopenia. Various hormones such as testosterone, estrogen, growth hormone, and creatine, angiotensin II converting enzyme inhibitors (ACEIs), anti-myostatin, specific androgen receptor modular (SARMs), and others were suggested for sarcopenia. Among them, creatine, ACEIs, myostatin are most commonly accepted medical therapeutic agents. Creatine from meat is potential ergogenic aid due to its buffering action against proton accumulation [29]. ACEIs works for skeletal muscle by suppressing the angiotensin-aldosterone system, reducing the pre- and afterload on the heart improving myocardial contractility Although the mechanisms of ACEIs are still not clear, it is obvious that ACEIs can improve cardiac output and improve blood flow to muscle, reduce inflammatory cytokines, etc [30-31]. Myostatin play important role as negative regulator of skeletal muscle myogenesis [32-34]. There are numerous potential targets for increasing muscle mass and strength, and the development of new medication for sarcopenia represents a possibly exciting clinical field. A better understanding of the molecular changes behind sarcopenia will help researchers develop better therapies to improve the skeletal muscle of elderly persons.
Physical Exercise
Successful aging is highly related to physical fitness levels [35]. Exercise is also very important factors to prevent sarcopenia [36]. Resistance exercise increases muscle mass, muscular strength, and physical function, whereas aerobic exercise improves cardiopulmonary capacity. Recent evidence on resistance training supports earlier research that it may be the most effective strategy to combat sarcopenia though muscle hypertrophy and increased muscular strength and power [37]. In addition, research has focused on the impact of resistance training on muscular strength rather than power, which is the product of force and speed [38].
Borde et al. (2015) reported that when exercising with an intensity of 70-79% of 1RM, it is the most effective for improving muscle strength [39]. Jung et al. (2019) also reported that high-intensity circuit training improves muscle mass and strength, body composition, balance and pulmonary function in sarcopenia patients [40]. Therefore, it is necessary to continuously explore effective exercise components in addition to recognizing and applying the importance of resistance exercise to the elderly with sarcopenia [41]. The effectiveness and outcomes of this resistance exercise depends on intensity, sets by repetitions, frequency, resistance type, and periods of training. Recent evidence from meta-analysis has revealed that resistance exercise may be actual in enhancing not only appendicular muscle mass, but also knee extension strength and timed up and go in elderly diagnosed with sarcopenia [41].
The endurance exercise increases the maximum aerobic capacity, so when constructing an exercise program for frail older people, and appropriate balance of endurance and strength exercises is necessary [42-43]. Falls in older adults under the influence of sarcopenia also emphasize the importance of exercise, and in addition to strength and power, a balance exercise based on ability is recommended [44-46]. However, since an exercise program suitable for the elderly should be considered to safety, a group exercise that can be supervised and encouraged is recommended [47]. In addition to this, steady frequency, sufficient warm-up, short-term or long-term goals, and nutrition should be considered essential components [48].
Conclusions
Sarcopenia is an emerging problem considering the rapid growth of the elderly population these days. To reduce the socioeconomic, it is critical to focus on sarcopenia, which is aging related condition resulting in skeletal muscle loss. Pathophysiology illustrates that sarcopenia is strongly associated with gerontology and its accompanying problems. A multidisciplinary approach including nutrition, exercise and medical management are essential to approach effectively treat this emerging condition. In addition, exercise intervention is an economical and cost-effective method of prevention, so exercise specialists need to understand the characteristics of sarcopenia and construct exercise programs. In a future study, it is necessary to find a more effective exercise program by applying various programs introduced as exercise intervention for the elderly and comparing them with the diagnostic criteria of sarcopenia.