Effect of Jujubae Fructus on Fatigue Recovery in Mouse Exercise Model
Article information
Abstract
OBJECTIVES
Jujubae fructus (JF, Ziziphus jujuba var. inermis Rehder) is an economically and agriculturally significant perennial fruit tree crop of the Rhamnaceae family and is used as a traditional herbal medicine. However, the anti-fatigue effect of JF has not been reported yet. The purpose of this study was to examine the anti-fatigue effect of JF by in vivo experiments.
METHODS
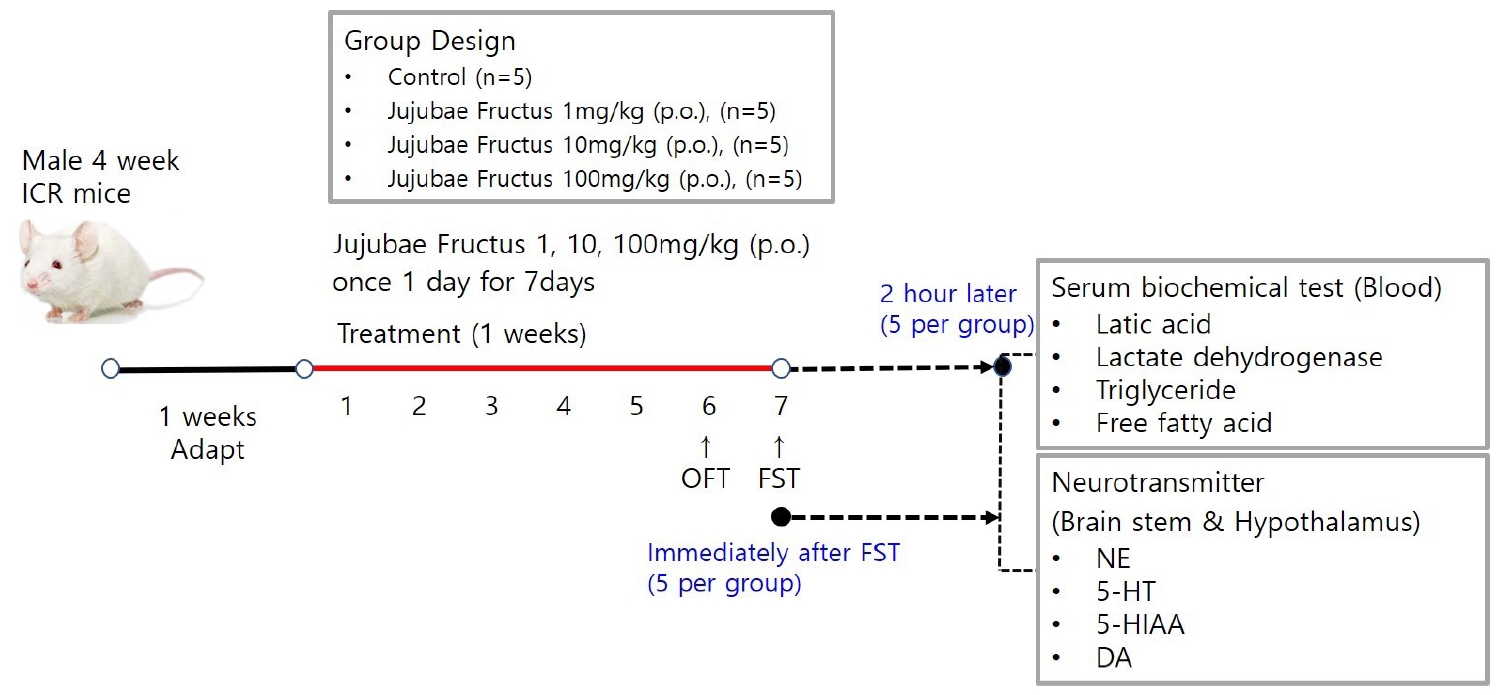
Male ICR mice were classified into a saline-treated swimming load test group (control) and JF (1, 10, 100 mg/kg) supplied swimming load test group (JF), and randomly assigned to 5 mice per group. Distilled water and JF were orally administered. The anti-fatigue effect of JF was evaluated by open field test (OFT) and forced swimming test (FST).
Results
JF significantly increased the total distance moved in OFT in a dose-dependent manner. JF was significantly decreased immobility time compared to control group in the FST. The increase in lactic acid and lactate dehydrogenase after FST was suppressed by JF treatment. Consumption and recovery of energy sources (free fatty acid and triglyceride) were improved after FST through JF treatment. Administration of JF decreased the expression of neurotransmitters such as norepinephrine (NE), serotonin (5-HT), 5-hydroxyindole-acetic acid (5-HIAA), dopamine (DA) by FST in brain stem and hypothalamus.
Conclusions
Based on these results, this study provides scientific proof for anti-fatigue effect of JF and it could be a useful material.
Introduction
Fatigue, caused by extreme physical and/or mental weariness and exhaustion, is one of the common and serious physiological problems resulting from severe stress, hard physical or mental work [1]. Fatigue is associated with many disorders and mainly caused by the depletion of energy sources which include the accumulation of lactic acid initiated by anaerobic metabolism of glucose in liver [2]. Physical fatigue is defined as the transient inability to maintain voluntary physical activities, which is considered to be related to deterioration in performance caused by intense physical exercise [3]. Numerous biochemical parameters, including lactic acid (LAC), lactate dehydrogenase (LDH), triglyceride (TG), glucose and free fatty acid (FFA) are involved in exercise [4].
The causes of fatigue are related to physiological and biochemical changes in muscles and homeostasis changes in the central nervous system [5]. Central fatigue is fatigue perceived in the central nervous system, and is caused by changes in neurotransmitters such as serotonin, dopamine, and prolactin in the central nervous system. When body temperature rises through exercise, serotonin activity increases, resulting in functional damage and fatigue in the central nervous system, resulting in loss of will to exercise [6]. In addition, the synthesis of serotonin is affected by changes in the concentration of free tryptophan in the blood, which is a precursor, and free tryptophan is changed by free fatty acids used as an energy source during exercise. In other words, increased free fatty acid due to exercise increases the concentration of free tryptophan in the blood, thereby increasing free tryptophan flowing into the brain, thereby causing central fatigue with increased serotonin synthesis in the brain [7]. Dopamine is a neurotransmitter secreted from the terminals of nerve fibers. It is a small molecule that reaches target cells through synapses and spreads to neighboring cells. It binds specifically to dopamine receptors distributed in nerves. Impaired transmission of excitation between nerves affects brain arousal [8]. In stress hormones, short-term stress causes the secretion of epinephrine and norepinephrine, which are called catecholamines, and stimulation of the sympathetic nerve, resulting in increased heart rate, increased blood pressure, and increased alertness and vigilance. Long-term stress stimulates the adrenal cortex to secrete cortisol. It increases blood pressure and blood flow, breaks down proteins and fats, and increases energy production [9]. As such, hormones secreted against the stress of exercise are transported to human tissues through the blood and respond to given stimuli, and hormones involved in stress respond sensitively to physical and mental stress and respond quickly to external stimuli. and is involved in regulating metabolism [10].
Chronic fatigue, which is characterized by persistent fatigue, can cause serious health problems, so control of fatigue is becoming an important issue in modern society. Modern people who are in a situation where it is difficult to avoid excessive work in a modern society with a lot of work and stress are forced to suffer from physical damage and fatigue. In order to improve this, dietary supplements for improving exercise performance, which had been consumed only by athletes in the past, became a necessary time for the general public. Dietary supplements are preferred, but they are difficult to ingest because of the high price and difficulty in verifying efficacy. As a result, fatigue improvement methods using natural products are emerging as an alternative.
Jujubae fructus (JF), herbal plant used in traditional oriental medicine, belongs to the Rhamnaceae family [11]. The fruits are very popular in world wild for nutraceutical food and are also used as flavoring agent. The phytochemical and pharmacological studies have shown that the active compounds are present in leaves, bark, fruit and seed of the plant. The main biologically active components are vitamins C and E, flavonoids, phenols, triterpene acids, polysaccharides and saponins [12]. Recent, JF have been reported to bring various pharmacological effects, such as anticancer [13], gastroprotective effect [14], antigenotoxicity [15], etc. However, the anti-fatigue effect of JF is yet to be reported.
This study evaluated the fatigue recovery properties of JF in mice by performing in vivo experiment, including open field test (OFT) and forced swimming test (FST) after oral administration of JF. In addition, in order to investigate the effect of JF on fatigue recovery, the effect of fatigue recovery was investigated by measuring locomotor activity, immobility time, biochemical serum analysis, and neurotransmitter expression.
Materials and Methods
Preparation of Jujubae Fructus
JF was purchased in the Omniherb (Yeongcheon, Korea). JF (200 g) was extracted with 1 L distilled water for 150 min at 100℃, and filtered through Whatman No.2 filter paper (Whatman Ltd., England). The extracts were concentrated in a vacuum using a rotary evaporator and then were freeze-dried at -80℃. The JF extracts were stored at -20℃ during experiment. The yield of the dried extract of JF was about 33.84%. JF was administered orally to mice once a day for 2 weeks. All experiments were carried out 1 h after administration of JF.
Animals
Male 4-week-old ICR mice (21-23 g) were purchased from Daehan Biolink (Eumseong, Korea). Mice were housed individually in the home cages in a controlled room temperature (22±2℃) and humidity (50±5%) with a 12 h light-dark cycle (lights on at 7 a.m.). Standard food pellets and tap water ad libitum were allowed in mice. After adaptation for 1 week, all experiments were carried out. Control group (a swimming group after swallowing saline, n=5) and JF (1, 10, 100 mg/kg, n=5 per group) group (a swimming group after swallowing JF) were used in one experiment. Due to sacrifice of mice, all experimental procedures at 0 and 120 min after FST were performed separately. All experimental procedures were repeated three times. This protocol was approved by the Institutional Animal Care and Use Committee of Daegu Haany University (Approval number: DHU 2021-096).
Open Field Test
Mice were dosed with JF (1, 10, 100mg/kg) by concentration and placed in a black square acrylic instrument (size range 25cm × 25cm × 30cm). The total distance moved was measured with the ethovision system after moving freely without interruption for 20 minutes. The total distance moved by group was measured at 5-minute intervals to confirm the continued increase in voluntary activity.
Forced Swimming Test
Control group (saline) and JF (1, 10, 100 mg/kg) group for 2 weeks were each subjected to a swimming adaptation training twice a week. Experimental animals adapted for 2 weeks were fed only with water for 16 hours before the forced swimming test and fasted for fast swimming test. Approximately 70% of water is added to the acrylic plastic water tank (70 × 70 × 60 cm) at a temperature of 24℃ to 26℃, and the weight corresponding to 5% of the weight is suspended by the tail of the mouse according to method of Leichtweis et al. [16]. If the mouse did not float to the surface for 7 seconds in water, it was judged burn-out. After forced swimming, immobility was measured. Based on Abdul's method [4], a typical immobility judgment was made such that the mouse only floated on the water, with only a small amount of movement in order to balance the body while only a part of the upper body including the face was exposed on the surface of the water. All experiments were conducted through blind test. When the upper body was submerged, it was declared exhausted. It was excluded from the floating time.
Biochemical Serum Analysis
After two weeks of experimentation, mice were fasted before sacrifice. Mice were lightly anesthetized, then all blood samples were collected from the aorta, kept on ice for 30 min, then centrifuged at 13,000 rpm for 10 min at 4℃ and stored at -80℃. The concentration of lactic acid, lactate dehydrogenase (LDH), triglyceride (TG), glucose and free fatty acid (FFA) were measured in the blood using a commercial assay kit (Sigma, MO, USA).
Measurement of Neurotransmitters
After FST, mice were sacrificed through cervical dislocation, brain stem and hypothalamus were collected quickly and neurotransmitters were analyzed. Brain stem was trimmed to a same size using vernier caliper then placed side by side on a frozen microtome stage and quickly frozen on OCT embedding medium Tissue-Tech using dry ice. Brain stem was collected in the same well, divided into corona planes through equivalent brain stem regions of the same thickness (60 μm) with sweeps of the same microtome blade. All dissection work was performed on glass plates filled with iced 0.01M phosphate buffered saline (PBS). All the collected tissues were rapidly frozen in liquid nitrogen and preserved at –80℃. Hypothalamic parts including anterior boundary of the optic chiasm, anterior boundary of the mamillary bodies, and lateral hypothalamic sulci was dissected. The dissection was about 3 mm deep. After removal, tissues were exercised by cutting off the abdominal aorta and washing it with PBS. Following the manufacturer’s protocol, levels of norepinephrine (NE), serotonin (5-HA), 5-hydroxyindole-acetic acid (5-HIAA), dopamine (DA) were measured in brain stem and hypothalamus using ELISA kit (MybioSource, San Diego, CA, USA).
Statistical Analysis
All data are expressed as the means±standard error of mean (SEM). The statistical significance of the differences between the groups were analyzed using one-way analysis of variance (ANOVA) followed by Newman-Keuls test in GraphPad Prism (version 5.03). Value of P<0.05 was considered statistically significant.
Effect of Jujubae Fructus on Locomotor Activity in Open Field Test
As shown in <Table 1>, JF 10 and 100 mg/kg significantly increased total moving distance compared with control group (P<0.05). At 20 min, similar tendency was observed in JF 100 mg/kg group (P>0.05).
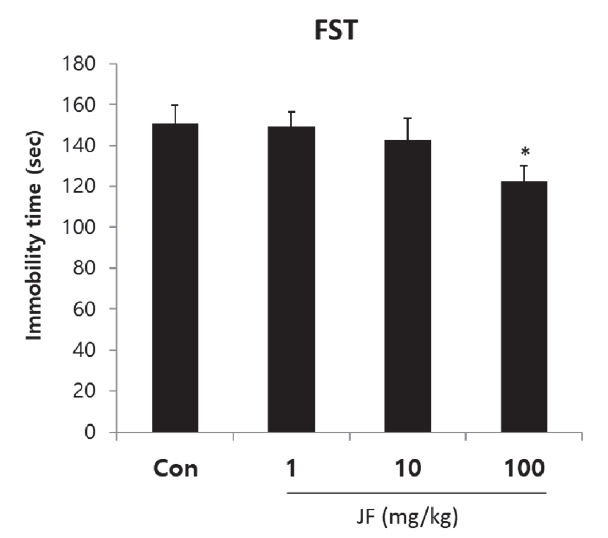
Effect of Jujubae Fructus on Immobility Time in Forced Swimming Test
The effect of JF on the forced swimming time is shown in <Figure 3>. Immobility time of JF 100 mg/kg group was significantly lower than that of control group (*P<0.05).
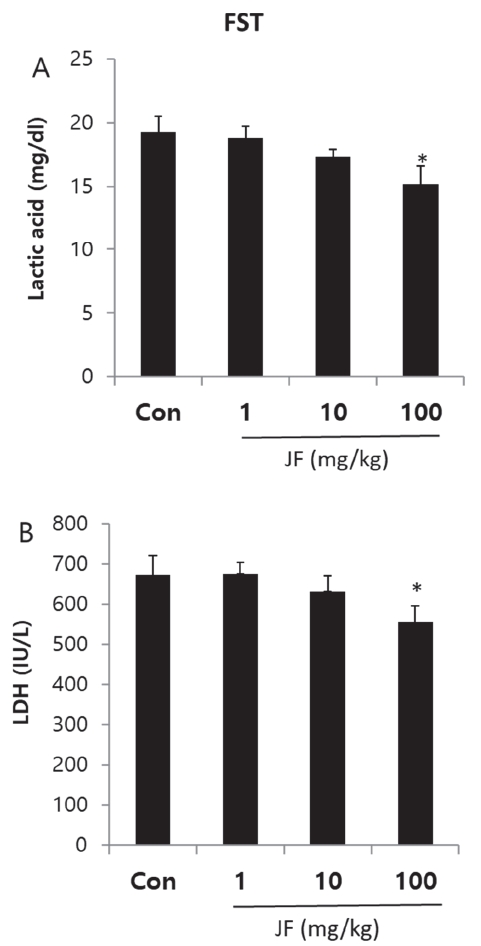
Effect of Jujubae Fructus on Fatigue-related Biochemical Markers after Forced Swimming Test
As shown in <Figure 4>, lactic acid and LDH levels were significantly increased by FST, which were significantly attenuated by JF (100 mg/kg) group (*P<0.05).
Effect of Jujubae Fructus on Free Fatty Acid and Triglyceride after Forced Swimming Test
As shown in <Figure 5>, FFA and TG levels were decreased immediately after FST by JF treatment compared to control group. After 120 minutes of rest, TG level was significantly increased in JF (100 mg/kg) group compared to control group(*P<0.05).
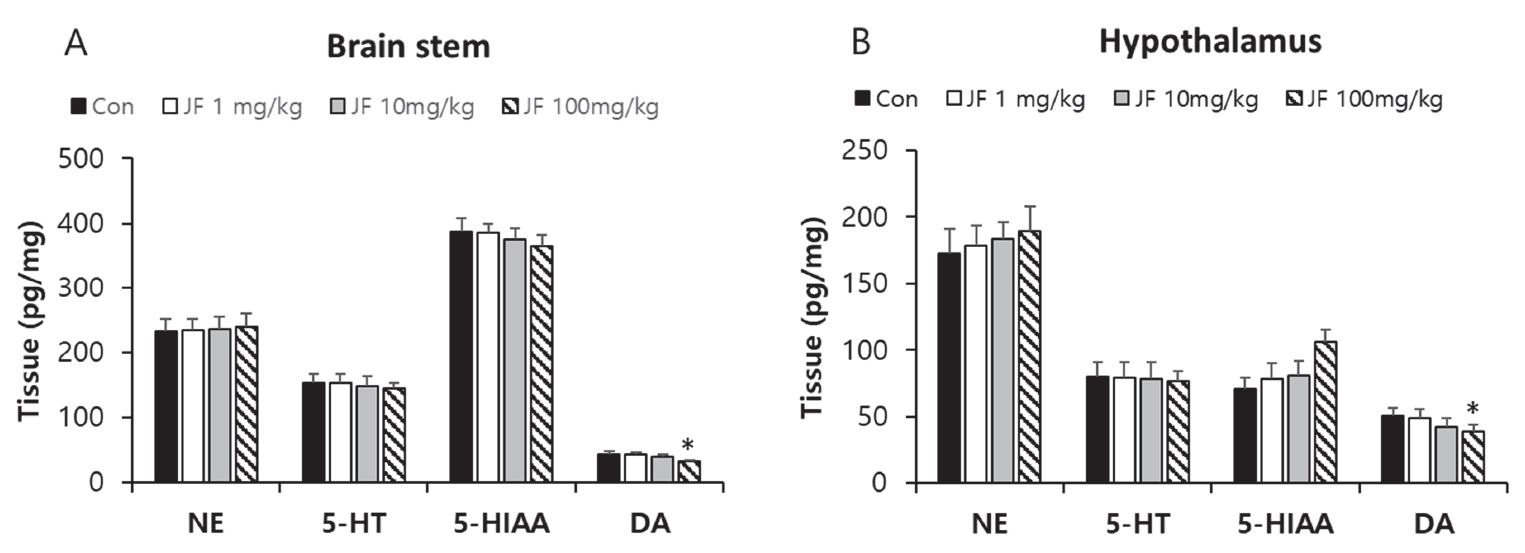
Effect of Jujubae Fructus on Levels of Neurotransmitters in Brain stem and Hypothalamus Tissue of Mice
When JF (100 mg/kg) was administered orally, 5-HT and 5-HIAA levels were lower in brain stem compared with control group <Figure 6A, P<0.05>. In addition, oral administration of JF (10 and 100 mg/kg) resulted in a lower level of NE, 5-HT, and DA in hypothalamus compared with control group <Figure 6B, *P<0.05>.
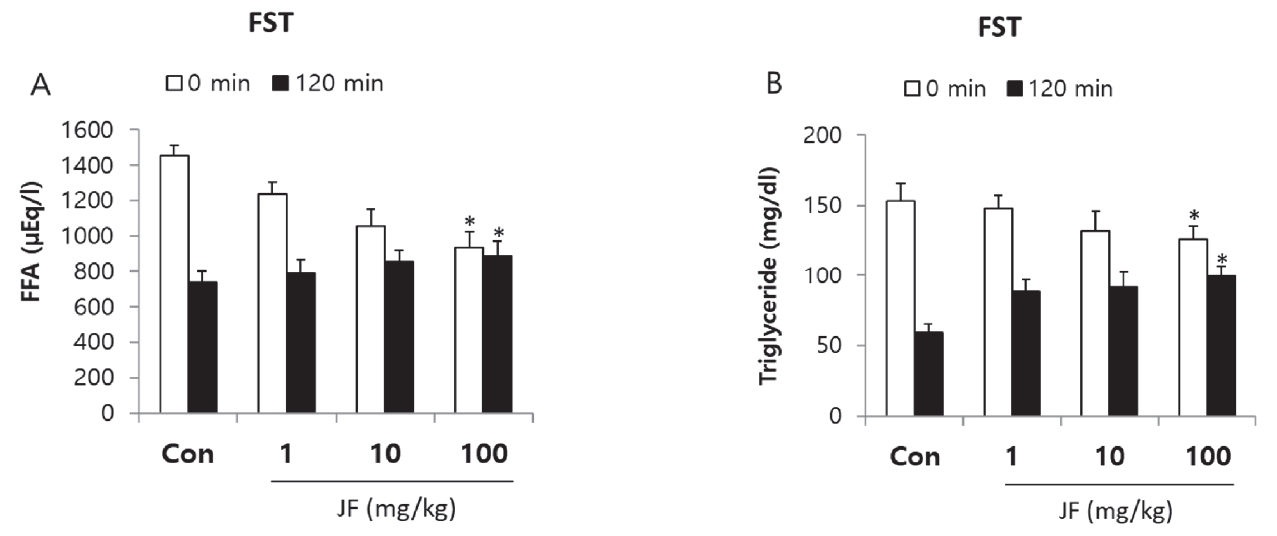
Effect of JF on Serum Level of FFA and TG after FST.
Notes: JF: Jujubae Fructus; FST: Forced swimming test. *P<0.05 vs. control (0 mg/kg) group.
After 120 min of FST, as compared with the control group expression of DA significantly reduced in the JF (100 mg/kg) groups in brain stem and hypothalamus tissues <Figure 7, *P<0.05>.

Effect of JF on Expression of Neurotransmitters in Brain Stem and Hypothalamus Tissue of Mice in 0 min.
Notes: JF: Jujubae Fructus; FST: Forced swimming test. *P<0.05 vs. control (0 mg/kg) group.
Discussion
Fatigue from excessive damage to the body causes various disorders, which can ultimately damage the nervous system, endocrine system, and immune system [17]. Physical fatigue due to excessive work or exercise and mental fatigue due to lack of sleep are also leading causes of poor quality of life [18]. In particular, physical fatigue can lead to chronic fatigue if lactic acid accumulates excessively due to depletion of energy sources, so prevention or quick treatment is needed. Lactic acid is produced during high-intensity exercise to produce sufficient energy, regulating the pH of muscles and blood, and inducing various damages such as oxidative stress [19]. The forced swimming load model is a representative animal model of excessive body damage, and similar phenomena occur, such as depletion of energy sources like humans and increased fatigue factors.
JF is a traditionally popular food and medicine in Asia [20]. The present study shows JF had an anti-fatigue activity in the OFT and FST model. Fatigue is one of the common physiological reactions occurred from exercise, depression, and aging [20]. The OFT and FST are the most simple and accurate models for evaluation of anti-fatigue activity in animals. Continuous use of muscle depletes ATP, an energy source in the muscle, accumulates substances indicating muscle fatigue, weakening muscle strength, and losing athletic ability as well as decreasing endurance [21]. Therefore, it is very important to remove fatigue from muscles to improve endurance and maintain muscle strength. Fatigue is a physiological phenomenon that occurs after work or exercise [22]. The main causes of exercise fatigue are depletion of energy sources, accumulation of fatigue-related factors in muscles, lack of control over nerves, and loss of homeostasis [23]. FST is a representative animal model of excessive body damage, and similar phenomena such as depletion of energy sources and increase of fatigue factors appear kike humans [24]. Immobility time of mice increases significantly due to loss of motor ability after FST like humans <Figure 4>. As a result, compared to control group, JF increased motor activity and decreased immobility time <Table 1, Figure 4>. Continuous use of muscles depletes ATP, an energy source in the muscles, accumulates substances that indicate muscle fatigue, weakening muscle strength and losing exercise capacity [24]. Therefore, it is very important to remove fatigue substances in muscles in order to recover fatigue and maintain muscle capacity. Lactic acid in the blood is a typical fatigue substance that occurs during high-intensity exercise, and it is reported that rapid removal is important to remove or relieve fatigue [25]. Storage and supply of energy sources are important factors in motor endurance. Exercising without energy sources increases physical fatigue and greatly reduces athletic endurance [26]. Typical sources of energy are fat and carbohydrates. Therefore, if energy sources are limited, fatigue improvement agents should convert the triglycerides accumulated in fat into fatty acids in the body to enter the blood, thereby enabling energy generation. Immediately after exercise, high serum levels of free fatty acids and triglycerides indicate that energy sources are depleted. However, after recovery, it can be seen that fatigue is improved when the serum levels of free fatty acid and triglyceride are high for recovery. Recovery from fatigue requires accelerating elimination of the metabolite accumulated in the body, Lactic acid is metabolic product of anaerobic glycolysis during intense physical exercise. LDH, cytosolic enzyme, is involved in anaerobic glycolysis and gluconeogenesis, which catalyzes lactic acid to transform into pyruvate. Levels of fatigue-related biochemical markers in serum, lactic acid and LDH were measured after last FST. Fat metabolism is related to late phase of exercise, can be an index of maintenance of exercise endurance. To evaluate whether the effect of JF after FST are associated with energy consumption, FFA and TG levels were measured. Exercise-induced fatigue such as forced swimming can be evaluated with biochemical indicators, such as lactic acid, LDH, FFA and TG in serum given forced swimming and JF treatment for 2 weeks. Lactic acid is used a major indicator of muscle fatigue. Intense physiology exercise leads to accumulation of lactate result in generation of fatigue [27]. Lactate dehydrogenase is an indicator of muscle damage [1]. In this study, JF group showed a dose-dependent decrease in serum levels of lactic acid and LDH after FST <Figure 3>. It may suggest that decreased immobility time in FST is associated with lactic acid and LDH. In this study, FFA and TG levels of JF group were increased immediately after FST compared control group. After 120 minutes, TG level of control group was decreased compared JF group <Figure 4>. Taken together, these findings suggest that jujube may ameliorate fatigue via regulation of serum lactic acid, LDH, FFA and TG level in FST animals.
Neurotransmitters are chemical messengers that transmit signals from neuron to synapses. Major neurotransmitters including NE, 5-HT, 5-HIAA, and DA are regulated by exercise and may affect brain function [28,29]. In general, upregulation of NE, 5-HT, 5-HIAA, and DA after drug administration is considered an anti-depressant effect. In vivo experiments have reported increased levels of NE, 5-HT, and DA in the brain and hypothalamus after various types of exercise [29,30]. Upregulation of these neurotransmitters is beneficial to the body, but excessive release of these neurotransmitters causes side effects such as muscle cramps [31]. Especially, 5-HT and DA imbalances in the brain are associated with fatigue induction, and several clinical researches support the serotonin hypothesis that increased 5-HT levels lead to fatigue [32-34]. Additionally, recent studies have reported that DA imbalances such as excessive or insufficient DA levels in the brain, cause fatigue [35]. In this study, administration of JF decreased the expression of neurotransmitters such as NE, 5-HT, 5-HIAA, and DA after exercise. In Particular, after exercise and rest, the expression of DA was significantly decreased in brain stem and hypothalamus <Figure 5 and 6>. The anti-fatigue effect of JF may come from inhibition of the dopaminergic, noradrenergic, and serotonergic systems.
Conclusions
In present study, JF group increased total moving distance and significantly reduced immobility time compared control group. JF group showed significantly decrease in serum lactic acid and LDH levels, compared to control group. In JF group, serum levels of FFA and TG immediately after FST are decreased dose-dependently, while serum levels of FFA and TG 120 min after the end of FST are increased. Neurotransmitter expression results indicate that oral administration of JF could ameliorate fatigue and improve exercise tolerance by inhibiting brain monoamines, including NE, 5-HT, 5-HIAA, and DA, in forced swimming test model. Based on these results, this study provides scientific proof for anti-fatigue effect of JF and it could be a useful material.
Notes
The authors declare no conflict of interest.