Effects of Regular Aerobic Exercise on Cardiovascular Health Factors and Heart Function in Sedentary Male Office Workers
Article information
Abstract
OBJECTIVES
This study aimed to investigate the effects of regular aerobic exercise on cardiovascular health factors and heart function in sedentary male office workers.
METHODS
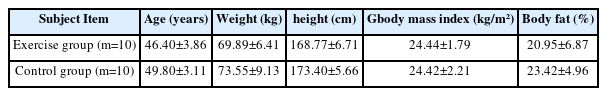
To achieve this aim, 20 individuals were selected as subjects: 10 in the exercise group (who have been regularly exercising for over 5 years, more than 4 times per week, for 1 hour or more per workout) and 10 in the control group (defines sedentary behavior more of moderate-intensity exercise for 30 minutes or longer, at least three days a week). For data processing, the average and standard deviation were calculated using the SPSS 21.0 statistical program, and the difference in the mean value change between groups was verified by performing an independent t-test.
RESULTS
There were significant differences between groups in factors related to cardiovascular health, such as resting heart rate, maximum systolic blood pressure, maximum oxygen intake, and exercise duration. Regarding heart function, there were significant differences between both groups in peak velocities of early and atrial fillings, as well as in early and late diastolic annulus velocities.
CONCLUSIONS
Taken together, regular aerobic exercise appears to have a positive impact on cardiovascular health factors and heart function in sedentary male office workers.
Introduction
With the recent advances in science and technology, medical technology and equipment have substantially developed, leading to an increase in preventive examinations for the early detection of diseases. However, despite the increase in early detection, the mortality rate due to cardiovascular diseases and metabolic syndrome-related diseases, which are decreasing in developed countries, is increasing quite rapidly in South Korea [1].
In addition, related measures such as social distancing due to the COVID-19 pandemic have further contributed to the spread of metabolic syndrome [2,3]. This increasing trend of metabolic syndrome has been reported to increase the risk of serious secondary diseases such as cardiovascular diseases [4], thus emphasizing the urgency of raising awareness [5]. In modern society, people spend a substantial amount of time sitting down, which has led to mounting interest in the consequent decline in physical activity, that is, a sedentary lifestyle. Therefore, it has been stated that exercise to strengthen muscles is an essential and indispensable factor in preventing the risk factors for musculoskeletal disorders caused by an increase in sedentary lifestyle [6]. A sedentary lifestyle is defined as any waking behavior with an energy expenditure of 1.5 metabolic equivalents (METs) or less while sitting or reclining. However, the American College of Sports Medicine (ACSM) defines sedentary behavior more broadly as a lack of moderate-intensity exercise for 30 minutes or longer, at least three days a week for a period of three months [7].
In other words, it not only includes a sedentary lifestyle but also physical inactivity [8]. Recent studies on sedentary lifestyles have reported that it is linked to not only chronic diseases but also a higher mortality rate [9,10] and has an adverse impact on physical health [11]. Particularly, work-related physical activity may occur repeatedly without allowing for adequate time to stabilize the increased cardiovascular function, and repetition of this phenomenon intensifies physical fatigue in a perpetuated vicious cycle [12]. Repeated sedentary behavior is thus more likely to result in the accumulation of physical fatigue, which in turn will increase the risk of cardiovascular diseases [13].
In 2023, the World Health Organization (WHO) reported that cardiovascular diseases cause 17.9 million deaths worldwide each year and that more than four out of five people die due to coronary heart disease; furthermore, 80% of these premature deaths were attributed to preventable and treatable risk factors such as an unhealthy diet and a lack of physical activity [14]. Most previous studies have reported that physical activity and aerobic exercise positively affect cardiovascular diseases and heart function [15-17], but another study reported that long-term aerobic exercise induces cardiovascular diseases and affects heart function, causing cardiac dysfunction [18].
To date, the amount of exercise that improves cardiovascular health and reduces accidents related to cardiovascular diseases has not been defined [19]. While the function and structure of the heart of people who have performed aerobic exercise for a long period of time have been reported to be deformed, depending on the period and type of exercise [20], such structural features of the heart are not necessarily clearly distinguished; furthermore, these structural features have been argued to vary depending on the intensity, duration, and frequency of exercise [21]. And in a study aimed at investigating the effects of regular aerobic exercise training on the electrocardiograms of middle-aged male patients with heart disease, it was found that changes in ST-segment during maximum exercise were consistent with a decrease in heart rate and heart rate ratio at autonomic exercise intensity at the same exercise intensity [22].
In other words, cardiovascular diseases and heart function seem closely related to the intensity and duration of exercise [23]. Therefore, aerobic exercise is highly recommended for cardiovascular health management and improvement. Among the many forms of aerobic exercise, swimming is considered one of the safest, with a lower risk of injury compared to land exercise [24], and it is considered to be the best choice for physical activity [25]. Swimming has been reported to reduce joint loads by reducing gravity through buoyancy [26], and it is effective against the risk of heart disease because it increases the venous return rate with a streamlined posture [19].
Exercise is considered one of the most important treatment methods for patients with cardiovascular diseases and participating in cardiac rehabilitation and aerobic exercise has great clinical significance [15]. Despite these advantages, most studies related to cardiovascular diseases and heart function have mainly included land exercise, with a scarcity of scientific evidence correlating with swimming [27]. Furthermore, the beneficial effects of regular exercise on reducing cardiovascular risks are well-established [28,29], but the most convenient and beneficial “dose” of exercise to achieve this goal remains unclear [30]. Therefore, this study aimed to investigate the positive and negative effects of regular health promotion behaviors on the cardiovascular health factors and heart function of middle-aged sedentary male office workers.
Methods
Subjects
All study subjects were healthy sedentary male office workers. The exercise group consisted of individuals who have been swimming regularly for over 5 years, more than 4 times per week, for 1 hour or more per workout, with an exercise intensity rating of perceived exertion (RPE) of 11–15 (Selected after surveying swimming lovers in S area through a questionnaire). The control group consisted of individuals who had not engaged in adequate physical activity, per the ACSM’s definition of a sedentary lifestyle. This study was approved by the Institutional Review Board of P University prior to the commencement of this study (PNU IRB/2015_31_HR). Those with diseases that could affect the heart were excluded from this study. presents the general characteristics of the subjects <Table 1>.
Measurements and Statistical Analysis
Metrics
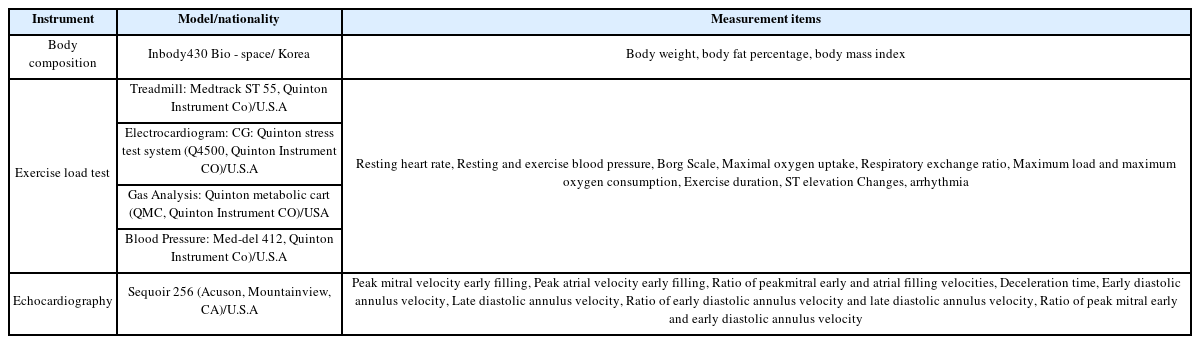
Body composition measurements (weight, body fat percentage, and body mass index), an exercise load test (resting and exercise heart rates and blood pressure (BP), rest and exercise electrocardiograms, cardiorespiratory fitness, myocardial ischemia, and arrhythmia), and echocardiography (heart function) were conducted as part of the study analysis.
Methods
Body composition
The subjects were asked to control their food intake for 3 hours before the study, rest for 30 minutes, and wear simple clothing without metal components before body composition measurements were performed. Their body weight, body fat percentage, and body mass index were measured using a body composition tester from Bio-Space.
Exercise load test
To test the exercise load, a treadmill exercise load test (Medtrack ST55, Quimtom, USA) was performed according to the Bruce Protocol. The subjects were asked to eat 5 hours before the experiment and rest for 30 minutes after arriving at the laboratory. The endpoint of the maximal exercise was set as the time when the heart rate did not increase anymore and was 17 or higher on the Borg Scale. The resting heart rate (HR) and resting and exercise BP were measured with a mercury sphygmomanometer. The maximum oxygen intake (VO2max), and maximum exercise time (minute) obtained by the exercise load test were recorded.
Echocardiography
Prior to measuring the heart function, the subjects were asked to rest for 10 minutes after arriving at the hospital. M-mode echocardiograms were recorded using a Sequoir 256 ultrasound machine and a 5-MHz transducer by a nurse specializing in echocardiography. Tissue-pulsed Doppler echocardiography was performed to examine the cardiac diastolic function by measuring the following mitral valve peak velocity variables: peak velocity of early filling (E), peak velocity of atrial filling (A), E/A ratio; and deceleration time (DT). Mitral annulus velocity variables, such as early diastolic annulus velocity (E’), late diastolic annulus velocity (A’), E’/A’ ratio, and E/E’ ratio, were also measured.
Data Processing
All data were analyzed by calculating the average value (mean) and standard deviation (SD) of the measured yield using the SPSS version 21.0 program. To verify the differences in average values between groups, an independent sample t-test was performed with the statistical significance level set at (α) = 0.05.
Results
Effects on Cardiovascular Health Factors
The resting heart rate (t=-4.200, p<0.01), resting diastolic BP (t=-4199, p<0.01), VO2max (t=4.111, p<0.01), and exercise duration (t=4.415, p<0.01) differed significantly between the two groups, but the MHR and RSBP were not significantly different. Furthermore, the MSBP and MDBP between groups were not statistically significantly different <Table 3>.
Effects on Heart Function
The A-velocity (t=-2.210, p<0.05), E/A ratio (t=-3.563, p<0.01), E’-velocity (t=3.477, p<0.01), and E’/A’ ratio (t=2.687, p<0.05) differed significantly between both groups, but the E-velocity and DT were not significantly different. Furthermore, the A’-velocity and E/E’ ratio between groups were not statistically significantly different <Table 4>.
Discussion
This study aimed to investigate and discuss the positive and negative aspects of swimming by analyzing the effects of regular aerobic exercise on sedentary male office workers’ cardiovascular health factors and heart function. While cardiovascular diseases account for a large portion of mortality and morbidity worldwide [31,32], as an intervention for the primary prevention of cardiovascular diseases, individuals are recommended to detect their long-term risks and manage their health [33,34]. The structural remodeling of the heart through exercise [35] is one of the major contributing factors to cardiovascular diseases [36], and regular physical activity and exercise have been reported to reduce the occurrence of cardiovascular diseases by lowering the resting heart rate [37]. In this study, the resting heart rate was close to bradycardia in the exercise group, which was considered a positive effect of aerobic exercise by previous studies [38]. It also seems to be associated with a positive rise in VO2max and ED.
Lower resting BP is a positive effect that can be expected from aerobic exercise because resting hypertension is a representative risk factor for cardiovascular diseases [39].
In this study, the exercise group had a lower resting BP than the control group, which was in agreement with a report that indicated that lowering BP through exercise by 4–9 mmHg is a positive effect of exercise [40]. Additionally, another study reported that short-term aerobic exercise positively affects BP [41].
Furthermore, the VO2max is considered the cardiovascular health factor on which aerobic exercise has the most important and positive effect, and it also acts as an important index for evaluating the prognosis of patients with cardiovascular diseases [42]. A higher level of VO2max was observed in the exercise group, which was in agreement with previous study results that indicated that aerobic exercise increased the recruitment of motor units by efficiently using oxygen in the peripheral muscles, prevented muscle fiber atrophy, and increased the VO2max [15].
In addition to the VO2max, the exercise time representing the ability to sustain exercise was the same as the VO2max. This was considered a natural outcome because an increase in the VO2max—which is the amount of oxygen consumed per 1 kg of body weight per minute and a measure of aerobic fitness [43]—led to an improvement in cardiorespiratory fitness and an increase in total body endurance [44].
Furthermore, although no statistically significant numerical results were found, the systolic BP during the maximum exercise of the exercise group shown in this study exceeded 200 mmHg, and there seemed to be concern regarding exercise-induced hypertension, with a 5- to 10-fold higher risk of leading to hypertension at rest [45], which was an independent risk factor for cardiovascular diseases [46]. Studies have reported that regular exercise significantly affects heart function by improving the level of cardiac autophagy, promoting the proliferation of cardiomyocytes, and reducing local tissue inflammation [47-49]. These changes in heart function have been reported to vary depending on the type, intensity, and time of exercise [49], and long-term aerobic exercise has been reported to increase the internal diameter of the left ventricle, thicken the septum and posterior wall of the ventricle, or cause eccentric hypertrophy without change [50, 51]. This eccentric hypertrophy is characterized by an increased ventricular internal diameter and a large left ventricular volume, resulting in a high stroke rate [52].
Therefore, the significant differences in peak velocity of early filling, peak velocity of atrial filling, and mitral annulus velocity in this study are considered to have been caused by eccentric hypertrophic remodeling through aerobic exercise. This is due to the mechanism by which the mitral valve opens and blood flows in when the pressure in the left atrium, which begins to rise immediately after myocardial contraction, is higher than that in the left ventricle [53]. In other words, the heart generally exhibits physiological and anatomical adaptations in response to chronic endurance exercise [54].
The peak velocity of early filling (E) was higher than the peak velocity of atrial filling (A). The E/A ratio is a value that reflects the myocardial relaxation function, and the normal range is 1 or higher. The numerical results of this study were also within the normal range for both groups. These results were consistent with the results of previous studies that analyzed cross-sectional comparisons between the exercise group and control group and reported an improved or normal E/A ratio in the exercise group [55-57].
However, another study reported that the higher E/A ratio in the exercise group compared to that in the control group was not clinically significant [58], which made it difficult to give meaning to the difference between the exercise and control groups, with all significant results between groups in this study being within the normal range.
Additionally, the DT is the time of mitral valve blood flow decelerating in the early diastolic period, and 220 m/s or less is considered normal. While a previous study reported that it was lower in the exercise group than in the general population [59], it was higher in the exercise group in this study, thus showing opposite results. However, the results of both groups were within the normal range, and no statistically significant results were found. In addition, the E’/A’ ratio, which is the ratio between the early diastolic annulus velocity (E’) and the late diastolic annulus velocity (A’), was higher in the exercise group, and a significant difference was also found between the groups. A similar preceding study reported that the exercise group that received endurance training regularly had a higher E’/A’ ratio than the control group [51], however, another study reported no difference in the E’/A’ among endurance athletes, resistance athletes, and Olympic (complex) athletes [60]. Therefore, this study’s findings suggest that, compared to other types of exercise, aerobic exercise can be expected to be relatively more appropriate because it results in the characteristics of eccentric hypertrophy with no clear pattern of myocardial deformation. However, further research on this topic is needed.
Finally, the E/E’ is the ratio of the peak velocity of early filling to the early diastolic annulus velocity, and it is a good indicator of left ventricular filling pressure. An E/E’ less than 8 was reported to be normal, but an E/E’ that is 15 or more was reported to be abnormal. Since the E/E’ was not affected by exercise [61,60], the results of this study showed no significant difference between the groups, and the values of both groups were less than 8 and within the normal range.
Conclusions
This study investigated the effect of regular aerobic exercise on cardiovascular health factors and heart function in sedentary male office workers and drew the following conclusions. In terms of cardiovascular health factors, the resting heart rate, resting diastolic BP, VO2max, and exercise duration differed significantly between both groups, but the MHR, RSBP, MSBP, and MDBP between both groups were not significantly different. In terms of heart function, the A-velocity, E/A ratio, E’-velocity, and E’/A’ ratio between groups were significantly different, but the E-velocity, DT, A’-velocity, and E/E’ ratio between groups were not significantly different.
These results suggest that even for variables that had not shown statistically significant results, the exercise group showed positive effects on all numerical values. This indicates that regular aerobic exercise positively affects cardiovascular health factors and heart function in sedentary male office workers.
Acknowledgements
The authors received no financial support for this article.
Notes
No potential conflict of interest relevant to this article was reported.