Neither Aerobic Interval nor Circuit Resistance Exercise Acutely Enhance Glucose Tolerance in Healthy, Young Adults
Article information
Abstract
Physical activity and glycemic control are important factors in preventing chronic disease such as Type II diabetes. Glycemic control may be enhanced during recovery from acute exercise.
OBJECTIVES
To test the effects of resistance exercise (RT) vs. aerobic interval exercise (AER) on post-exercise blood glucose (BG) control during an oral glucose tolerance test (OGTT).
METHODS
Ten volunteers completed three separate trials (counter-balanced order): a resting control trial (CON) consisting of a 75-min OGTT following consumption of a 25% glucose solution dosed at 1 g/kg body mass; a RT trial in which subjects completed a 30-min circuit protocol (6-7 sets) of 6 reps/set using 10-RM load for squat, bench press, knee extension and biceps curl; and the AER trial where subjects alternated between treadmill exercise (3 min) and arm crank ergometry (2-min) over a 30-min period (intensity target of 15 on the Borg 6-20 point Rating of Perceived Exertion scale). Both exercise trials were followed by the same 75-min OGTT. BG was assessed via fingertip sampling prior to exercise, mid-exercise, post-exercise, and every 15-min during the OGTT. Blood lactate was collected at rest, mid-exercise, post-exercise and at 15 min of the OGTT.
RESULTS
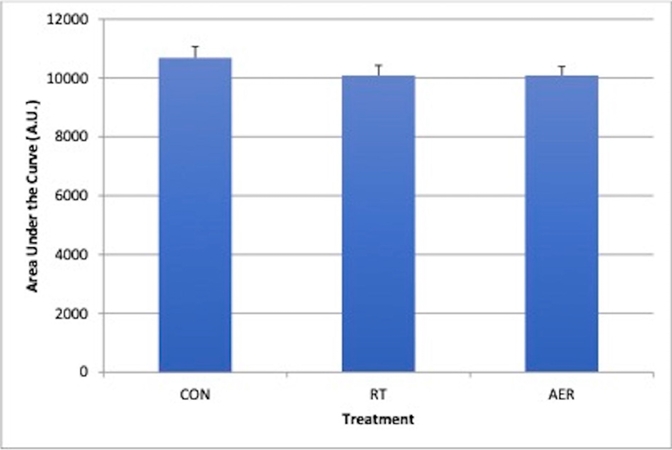
Both exercise trials elicited significantly increased lactate but were not different from one another. BG was significantly elevated during the OGTT for all conditions but was not different by condition. BG area under the curve (AUC) was 5.6% smaller (p>0.05) following RT and AER vs. CON (CON: 10687±381; RT: 10087±343; AER: 10093±299 arbitrary units).
CONCLUSIONS
Acute resistance and aerobic interval exercise were not found to elicit significantly enhanced post-exercise glycemic control. Post-exercise glycemic control may be related to the total energy deficit achieved in young, healthy adults.
Introduction
Based on the 2020 National Diabetes Statistics Report from the U.S. Centers for Disease Control and Prevention (CDC), 34.2 million individuals (10.4% of the population) in the U.S. had diabetes [1]. An additional 88 million adults (34.5% of the U.S. population) had prediabetes [1]. The CDC also reports that diabetes, a metabolic disorder in which the body’s ability to self-regulate blood glucose is impaired, will develop in 4 out of every 10 adults in the United States [1]. Chronic physical activity is associated with a reduction in all-cause mortality along with a reduced chance of dying from diabetic complications [2]. Given the prevalence of disordered glucose regulation and its associated health consequences, it is important to evaluate the role that acute exercise may play in potentially promoting transient improvements in glycemic control. Proposed glucoregulatory benefits of acute exercise include upregulation of glucose transporters (GLUT4) in the muscle cell membrane [3-5] and in peripheral blood mononuclear cells [6], improved insulin sensitivity [7,8], improved insulin independent glucose uptake [3,7-9] and a reduction in fatty acid intermediates within muscle [4,8]. Further, acute exercise also promotes an increase in glycogen synthase activation [7]. All of these factors may be of benefit in promoting the body’s ability to manage a glucose challenge during recovery from acute exercise.
It is widely accepted that aerobic exercise elicits a higher caloric expenditure rate in comparison to resistance exercise performed at the same relative intensity. The sustained activation of large muscle groups that occurs with aerobic exercise allows for a substantially greater energy expenditure rate. This may translate into a larger deficit in muscle glycogen stores, causing the mechanisms of blood glucose uptake to facilitate a greater clearance of blood glucose in comparison to resistance exercise. However, to the best of our knowledge, no studies have contrasted aerobic and resistance exercise effects on glucose clearance following exercise. To date, most research examining post-exercise glucose regulation has employed chronic exercise training. These studies often reflect an enhanced glycemic control during recovery from exercise of approximately 5-15% [10-12]. Resistance exercise protocols [13-16] have also revealed benefits for post-exercise glycemic control. Notably, the majority of these studies examined glycemic control hours to days following an exercise bout. Few studies have assessed glycemic control immediately or shortly after an exercise bout [17-20].
The study was designed to determine the independent effects of aerobic interval and resistance exercise on acute regulation of blood glucose following the specified mode of exercise. Aerobic intervals were selected to mimic the metabolic and perceived stress of a resistance training session. As resistance training often incorporates upper and lower body muscle groups, we chose to alternate between treadmill jogging and arm crank ergometry to employ upper and lower body musculature as well. To assess the effects of these exercise modes on glycemic control, an oral glucose tolerance test (OGTT) was supplied 10 min post-exercise. It was hypothesized that resistance exercise (incorporating upper and lower body musculature) and aerobic interval exercise (using arm crank ergometry and treadmill running to also incorporate upper and lower body musculature) would reduce the glucose area under the curve during recovery from exercise in comparison to a resting control OGTT trial. This would signify an enhancement of post-exercise glucose control resulting from a single acute aerobic interval or resistance training session of equivalent duration and comparable metabolic stress. This investigation is novel as it is the first to examine effects of full body, circuit-type exercise on post-exercise glucose control.
Methods
Ten, resistance trained volunteers (7 male and 3 female) were recruited to participate in the study. Volunteers had a minimum of 6 months of resistance training experience (3 or more days. week-1). Following collection of written consent documentation using institutional review board approved forms, demographic (mean ± S.D.) characteristics (age = 21.7 ± 2.31 yrs.; mass = 79.5 ± 12.3 kg; height = 175.9 ± 9.0 cm; % fat = 16.3 ± 5.4; BMI = 25.5 ± 2.5 kg.m-2) were obtained (Approval #1683). The subjects then completed baseline testing to establish load setting for experimental trials. Baseline testing consisted of determination of 10-repetition maximum (10-RM) for four resistance exercises (back squat (SQ), knee extension (KE), bench press (BP) and preacher’s biceps curl (BC)). Following these assessments, subjects had work rate (for arm crank ergometry) and running speed (for treadmill) determined. For these assessments, work rate (W) or run speed (mph) was gradually increased to establish a Rating of Perceived Exertion (RPE) of 14-15 (Borg 6-20 scale) [21]. Subjects were also introduced to indirect calorimetry instrumentation (ParvoMedics TrueOne 2400, Provo UT) for the aerobic interval experimental (AER) trial (described below). None of the participants were experienced with arm ergometry.
Experimental Trials
Each participant completed three experimental trials, all performed in a counter-balanced manner: 1) resting control trial (CON); 2) resistance exercise trial (RT); and 3) aerobic exercise interval trial (AER). Each trial consisted of an overnight fast prior to arrival at the lab for testing. Trials were conducted between 0800-1100 hrs. Upon arrival at the laboratory, subjects rested for 10 min in a seated position. Resting measurements for heart rate (HR) and blood pressure (BP) (Omron Intellisense Automatic Blood Pressure Monitor, Omron Healthcare, Inc., Bannnockburn IL), blood glucose (Bayer Contour, Bayer Inc., Leverkeusen Germany) and blood lactate (Lactate Plus, Nova Biomedical, Waltham MA) were then obtained in the seated position. For the CON trial, subjects were subsequently administered the oral glucose tolerance test (OGTT) beverage, dosed at 1 g/kg of body mass using a 25% dextrose (Now Vitamins, Bloomingdale, IL) solution. Following complete ingestion of the beverage, a 75-min OGTT was conducted with serial finger-tip blood glucose measurements collected every 15 min. Upon completion of the OGTT, final resting measurements for HR and BP were obtained. BP measures were converted to mean arterial pressure (MAP) for analysis. The OGTT dose was held consistent across each trial for each subject.
For exercise experimental trials, following collection of resting measurements described above, the subject completed a 3-min cycle ergometry warm-up (Monark 828E, Vansbro Sweden) at 70 W. For the RT trial, subjects then completed a warm-up set (8 repetitions) for each lift using a load that was approximately 50-60% of the 10-RM load. The protocol involved completion of 6-7 sets (6 repetitions per lift per set) in a circuit style format using the 10-RM load for each lift. Six repetitions were established as a manageable target through pilot testing, given the volume of sets that were applied in the study. Lifts were performed in the following order: SQ, BP, KE, BC. Both the RT and the AER protocols were designed to span 30-32 min. Following completion of set 3, the subject was seated and BG (blood glucose) and BL (blood lactate) were obtained (Mid-Ex). These measures, along with HR and BP, were obtained again within 5-min of completion of the exercise phase of the trial, immediately prior to commencing the OGTT phase (Post-Ex). RPE was assessed following completion of set 3 and upon completing the final set for both exercise trials. Lactate was also assessed at the 15-min OGTT measure time point (referred to as OGTT15). HR and BP were assessed again upon completion of the 75-min OGTT period (End).
Target treadmill speed and arm crank resistance were determined during a preliminary orientation visit where subjects were advised to perform the exercises at an RPE of 15 (Hard). This intensity was selected to align with the perceived intensity of the circuit resistance exercise program. For the AER trial, following collection of resting measurements and after completing the cycle ergometry warm-up, as described above, the subject was placed on the treadmill (DesmoPro, Woodway USA, Inc., Waukesha WI). Run speed was established (range 8-13.1 km.h-1) and the protocol commenced (mean running velocity ± S.D.: 182.2±15.8 m.min-1). The AER protocol involved 3-min treadmill running followed by 2-min arm crank ergometry (watts: 64.5±11.9) with 30-sec transition between modes. This sequence was completed for five sets. Heart rate (Polar FT7, Polar Electro, Inc., Bethpage NY) and RPE (Borg 6-20 scale) were collected during the last 15 seconds of stage 3 and 5 for both modes of exercise. Expired gases were analyzed (oxygen uptake, minute ventilation, respiratory exchange ratio) during set 3 and set 5 using indirect calorimetry (TrueOne 2400, ParvoMedics, Salt Lake City UT). The nose clip and mouthpiece were supplied to the subject at the start of the TM portion of the set and gases were analyzed continuously until completion of the arm crank phase of the set. Upon completion of set 3 (Mid-Ex), after returning to the seated position, BG and BL were obtained. These measures, along with HR and BP, were obtained again within 5-min of completion of Set 5 (Post-Ex) prior to commencing the OGTT phase of the trial. As with the RT protocol, blood lactate was re-assessed at OGTT15. Final HR and BP measures were obtained upon completion of the OGTT trial (End). A protocol schematic can be viewed in <Figure 1>.
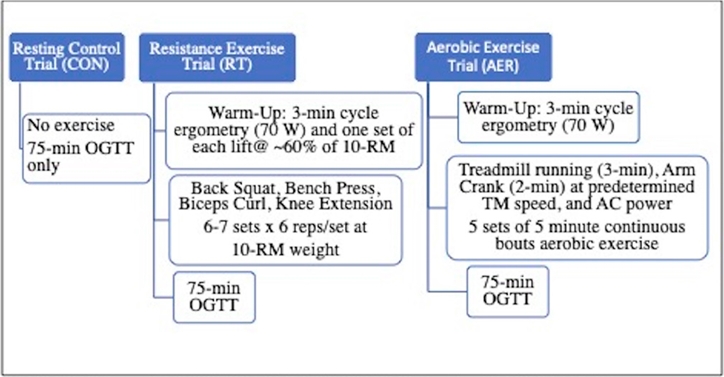
Experimental Model Schematic (TM: treadmill; AC: arm crank; OGTT: oral glucose tolerance test; RM: repetition maximum).
Blood glucose area under the curve (AUC) (arbitrary units: A.U) for the OGTT was determined using the trapezoidal method as:
Area = [(BG measure 1 + BG measure 2)/2] x 15
Where “15” represents the time (min) between adjacent samples (i.e., BG measure 1 and BG measure 2). Each of five midpoint derivations (from six sample points: immediately post exercise, and minutes 15, 30, 45, 60, 75 of the OGTT) was summed to derive the overall BG AUC for each test subject.
Statistical Analyses
Data were analyzed using repeated measures ANOVA (time x treatment) and one-way ANOVA for group analysis of BG area under the curve. Post hoc analyses using a Bonferroni test were employed to identify the location of significant time and interaction effects. Significant effects were accepted at an alpha level of p <0.05. All statistical analyses were conducted using R statistical software (R version 4.0.3) in RStudio (version 1.3.1093) using the rstatix and ez statistical packages (PBC, Boston MA). Results are reported as mean±S.E.
Results
Both exercise conditions resulted in significant increases in heart rate, RPE, mean arterial pressure (MAP) and lactate. For blood lactate <Figure 2>, time, treatment and time x treatment effects were present, such that Mid-Ex, Post-Ex, and OGTT15 measures for RT and AER were significantly greater than pre-exercise and significantly different from the CON trial (all p <0.05). No differences in lactate were present based on mode of exercise (RT vs. AER). RPE was significantly (p <0.05) elevated by exercise and was significantly greater for AER (13.9±0.4) vs. RT (12.2±0.5) Mid-Ex. Post-Ex RPE was not different by exercise mode (AER: 16.1±0.6 vs. RT: 15.2±0.6). Heart rate was significantly different between AER vs. RT (Treatment effect) and between both RT and AER vs. CON <Table 1>. Interactions were also present for HR, such that HR was significantly elevated by both modes of exercise relative to the CON trial at Post-Ex, and End <Table 1>. Set 3 and Set 5 HR in AER (173±3 bpm and 178±4 bpm, respectively) were significantly elevated above resting, Post-Ex and End HR. MAP was not different at Post-Ex but was found to be significantly lower at the final measurement (End) for both exercise conditions (p = 0.023). The MAP End measurement was not different based on mode of exercise <Table 1>. There were no significant treatment effects for blood glucose <Figure 3>. However, time effects were present such that BG prior to, during, and post-exercise was significantly different from all OGTT measurements. During the OGTT, 30-min and 45-min timepoints were significantly greater than all other measurement points. The BG AUC was 5.6% smaller for both exercise sessions vs. CON. However, the BG area under the curve (AUC) was not significantly different (p=0.276) between conditions <Figure 4>. No differences in the absolute rise in BG from Pre to the peak measure were observed (p = 0.414); though, the AER trial was associated with a 16.7% smaller rise in BG concentration relative to CON. RT was associated with a 7% smaller rise in peak BG concentration vs. CON. The OGTT30 BG concentration tended to be lower in AER vs. CON (p = 0.075). No other trends in BG responses were observed.
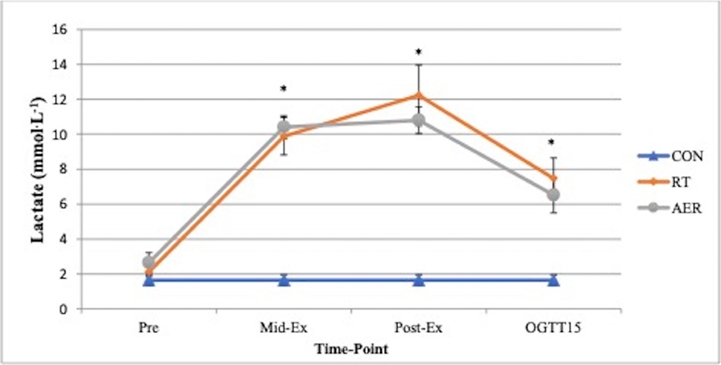
Blood lactate measures for Pre (baseline or pre-exercise), Mid-Ex (approximate mid-point of exercise trials), Post-Ex (immediately post-exercise) and OGTT15 (15 min time point after CHO ingestion). Conditions: CON = resting control trial; RT = resistance circuit training trial; AER = aerobic interval trial.
*Different from CON and Pre.
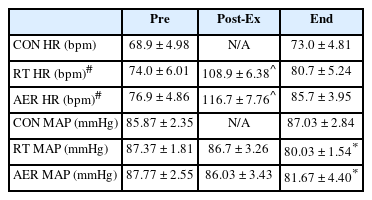
Heart rate (HR) and mean arterial pressure (MAP) responses (mean ± S.E.) before exercise (Pre), following exercise (Post-Ex) and upon completion of the 75-min OGTT period (End). CON measures were obtained before (Pre) and upon completion of the OGTT period (End).
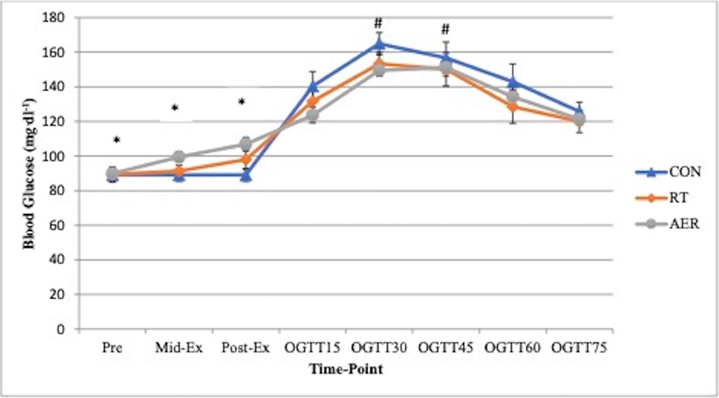
Blood glucose responses to the experimental conditions: CON = resting control trial; RT = resistance circuit training trial; AER= aerobic interval trial. For CON, the CHO solution was consumed after the baseline measurement (Pre). For exercise trials, the CHO solution was consumed after the post-exercise timepoint. OGTT: oral glucose tolerance test.
*Different from all time points; #different from OGTT15, 60 and 75.
Discussion
The study was conducted to examine the effects of full body, moderately intense exercise on acute, post-exercise glycemic control. In order to incorporate similar muscle groups, the aerobic interval portion of the study employed treadmill running and arm crank ergometry, while the resistance training portion of the study employed two upper and two lower body exercises administered in a circuit training style. The main finding of the study is that neither exercise condition resulted in a statistically significant improvement in glycemic control during the post-exercise OGTT. When contrasting BG AUC measures, exercise condition AUCs were 5.6% smaller than the CON AUC, but these were not significantly different. Similar reductions in BG AUC following acute exercise have been reported by other investigators [10-12,18,22]. Time effects were observed during the OGTT, but no group interaction effects were present. Of note, the 30-min OGTT glucose concentrations were 9.3% lower in AER (p=0.075) and 7% lower in RT (p>0.10) than CON. Similar magnitudes of exercise-induced BG changes have been reported by other investigators during OGTT testing [17].
Acute exercise has been associated with transient improvement in blood glucose regulation [12,15]. Improvements in glycemic control following exercise have been seen within hours up to days of an exercise bout [11,13]. While the benefits tend to be more prevalent in T2D subjects, healthy control subjects often also show improved glycemic control in response to a single exercise bout. The majority of work in this area has explored glycemic control in response to aerobic or resistance exercise. Earlier investigations studying post-exercise glycemic control have reported improvement in glucose clearance ranging from 5-10% [18,22]. In contrast, other investigators have not observed an enhancement of glycemic control immediately following exercise [17,19]. Arsa et al. [17] did not observe an attenuating effect of aerobic exercise on glycemia during an OGTT; however, when an additional 10-min light exercise session was added at the conclusion of the OGTT period, an improvement in glucose clearance rate was observed. Cook et al. [19] also failed to observe significant alterations in BG AUC, insulin response, or peak blood glucose during an OGTT administered immediately following concentric and eccentric treadmill exercise when compared to a resting control condition. Our findings align with these studies. Arsa et al. [17] employed a 20-min aerobic exercise session (moderate intensity) and Cook et al. [19] employed 40-min of treadmill exercise. It is possible that the volume of exercise was not sufficient to promote enhanced glucose clearance.
Several factors have been linked to the capacity of acute exercise to improve glucose regulation: duration and intensity of the exercise [18,23], level of muscle glycogen depletion [23], and the volume of active muscle [16,18]. The purpose of the present study was to contrast the effect of exercise mode (incorporating upper and lower body musculature under both exercise conditions) on post-exercise glycemic control. The AER protocol was designed to incorporate a broad muscle contribution by alternating between treadmill running and arm cycle ergometry. Similarly, the RT protocol was designed to incorporate upper and lower body musculature, with the aim of both protocols to provide a greater surface area for glucose disposal compared to the resting CON trial. Post-exercise glycemic control was assessed using a 75-min OGTT which commenced approximately 10 minutes after completion of exercise, with serial blood glucose measures obtained every 15 min.
AER work rate and resistive loads for RT were developed with the aim of placing a similar physiologic strain on the body while also matching session duration. Based on a variety of physiologic measures, including lactate, HR, and MAP, and a subjective measure (RPE), this objective was achieved. However, under this design, it was not practical to match total caloric expenditure. This operates as a limiting factor as it is likely that the AER trial led to a greater caloric deficit given the more continuous muscle activation that occurs with aerobic exercise. Cardiorespiratory measures were obtained during the AER trial only. Based on these assessments, average VO2 during the treadmill component was 2.88 ± 0.20 L.min-1 and during the arm crank component was 1.77 ± 0.13 L.min-1, with overall AER caloric expenditure measured at 11.63 ± 0.78 kcal.min-1 (~350 kcal total). The caloric cost of circuit style resistance training exercise has been estimated at approximately 8 kcal per minute [24] or 8 kcal/1000 lbs [25]. Based on these expenditure rate determinants, the RT protocol would equate to 173-240 kcal expended. So, it is presumed that the AER protocol created a substantially greater caloric deficit than the RT protocol. This potential energy expenditure difference, however, did not lead to different AUC responses for the two exercise conditions.
Resistance training programs have also demonstrated positive improvements in glycemic control. Andersson et al. [13] found that eight weeks of progressive resistance training in older adults led to a 14% improvement in glycemic control. The glucose AUC was significantly improved (34% lower) by training while no changes occurred in an age-matched control group [13]. The OGTT was conducted 48 h after the last exercise session [13]. So, these findings seem to be reflective of a training benefit. Fenicchia et al. [15] found a significantly smaller AUC (15%) when glucose tolerance was assessed 12-24 h after the first resistance training exercise bout (6-week training study). However, no difference was present when a follow-up OGTT was conducted 60-72 h after the last training bout [15]. Consequently, Fenicchia et al. [15] did not report a chronic training benefit as further improvement in glucose clearance was not observed. Acute exercise was found to lower the peak BG during the OGTT only in Type 2 Diabetic subjects in this study and not in age-matched controls [15]. Consequently, acute exercise may have a more profound impact on BG regulation in insulin resistant populations as opposed to healthy, young adults as used in the present study.
Bird and Hawley [24] reported that higher intensity exercise, such as high intensity interval training (HIIT), often improves whole-body insulin sensitivity and glucose transporter (GLUT4) activation. High intensity interval training (HIIT) studies have commonly been shown to improve glycemic control in healthy and Type 2 Diabetic participants. For example, a 12-week HIIT program was found to positively impact continuous glucose monitoring concentrations in T2D subjects [26]. Adding protein to the training sessions did not further enhance or impede these responses [26]. Lee et al. [10] conducted 12 weeks of HIIT in T2D adolescents and compared them to a low intensity exercise group (both creating 1200 kcal deficits per week). Glycemic control was significantly improved in both groups but was more potent in the HIIT group [10]. Shepherd et al. [11] also contrasted low volume HIIT exercise against moderate intensity exercise (MIE) over 10 weeks of training and found significantly better adherence with HIIT. Further, both modes resulted in significantly smaller BG AUCs (6% lower with HIIT and 4% lower with MIE) during the OGTT [11].
Bird and Hawley [24] suggest that resistance-based exercise promotes improved glycemic control and that the benefit may be optimized by the inclusion of aerobic exercise. In line with this premise, our protocol was designed to engage both upper and lower body musculature with both protocols. Given that post-exercise glucose regulation was not significantly improved compared to the CON trial, it is conceivable that glycogen deficits were not substantial enough to drive an enhanced glucose uptake. Consequently, it may take a longer exercise bout to manifest more potent improvements in glycemic control. Or, it may be that a delay in the OGTT trial might elicit different effects. However, a rationale for this prospect is not clear.
Timing of the post-exercise OGTT is widely variable in the literature. Numerous studies have shown that glycemic control is improved within 24 h [12,15,27,28] up to days [11,13,14] following exercise. It seems impractical that a delay of hours to days in administering the OGTT would result in a further enhanced glucose clearance rate compared to assessing clearance immediately following exercise, particularly since GLUT4s are believed to migrate from the membrane over the hours after exercise has been completed. There is also a greater risk of reduced control (both of diet and activity) when more time has elapsed between the last bout of exercise and administration of an OGTT. Consequently, the protocol in the present study was designed to begin the OGTT approximately 10 min after completion of exercise with the expectation that this might be the period of time when mechanisms in support of glucose disposal would be most active (i.e. increased insulin sensitivity and presence of activated glucose transporters). That glucose clearance was not significantly improved over the CON condition following exercise may be reflective, then, of insufficient muscle glycogen depletion. This may also be a limitation in the present study. Bird and Hawley [24] note that a dose response effect is often observed whereby insulin sensitivity is commonly improved when greater energy deficits and/or higher exercise intensities exist. In the present study, the exercise intensity of both protocols was reflective of significant effort based on lactate and RPE measures. The distribution of this effort across upper and lower body musculature would also mean that muscle glycogen use would be dispersed across more muscle area than had only a lower body protocol been employed with the same caloric deficits. This is an interesting consideration that would warrant further study. It is conceivable that a threshold for glycogen depletion might exist, beyond which point glucose uptake is heightened during recovery from exercise.
Conclusions
In conclusion, neither a single bout of RT or AER interval exercise was found to significantly reduce the 75-min BG AUC in response to an OGTT administered immediately following exercise completion. Though, there was a trend for a lower BG concentration at 30-min of the OGTT following AER exercise. Factors that should be considered when evaluating how acute exercise impacts glycemic control during recovery include: timing of the OGTT, volume of active muscle, and volume of energy deficit produced by the exercise session. In the present study, the total caloric deficit was approximately 350 kcal (AER) and roughly 200 kcal (RT). A more substantial energy deficit may be necessary for inducing enhanced post-exercise glycemic control in healthy, young adults. It is also recommended that research be performed to investigate differences in glycemic control that may result from different quantities of muscle involvement. Relevantly, the acute effects of exercise may be most pronounced in those with Type 2 diabetes. Ideally, more work should be conducted in this population to assess the potential benefits of acute exercise on post-exercise glycemic control.
Notes
The authors declare no conflict of interest.